S2201
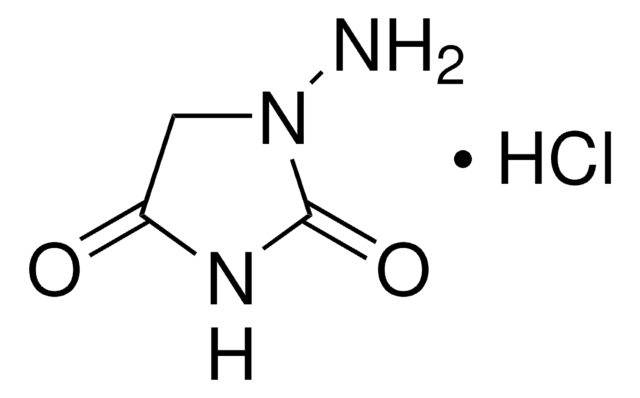
Semicarbazide hydrochloride
≥99%
Synonym(s):
N-Aminourea hydrochloride, Hydrazine carboxamide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
NH2CONHNH2 · HCl
CAS Number:
Molecular Weight:
111.53
Beilstein:
3593642
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
mp
175-177 °C (dec.) (lit.)
SMILES string
Cl.NNC(N)=O
InChI
1S/CH5N3O.ClH/c2-1(5)4-3;/h3H2,(H3,2,4,5);1H
InChI key
XHQYBDSXTDXSHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Derivatization reagent for aldehydes and ketones which produces crystalline compounds with characteristic melting points.
Semicarbazide hydrochloride is a general reagent used to synthesize semicarbazones from aldehydes and ketones. It can be used to build a variety of heterocyclic compounds, some of which are potent antimicrobial and antiviral agents. It can also be used to prepare corrosion inhibitors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Oral
Target Organs
Bone
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Semicarbazide.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors.
Goulart C M, et al.
Corrosion Science, 67, 281-291 (2013)
Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives.
Kirilmis C, et al.
European Journal of Medicinal Chemistry, 43(2), 300-308 (2008)
Design, synthesis, and biological evaluation of antiviral agents targeting flavivirus envelope proteins.
Li Z, et al.
Journal of Medicinal Chemistry, 51(15), 4660-4671 (2008)
Synthesis of some new azole, azepine, pyridine, and pyrimidine derivatives using 6?hydroxy?4H?4?oxo [1]?benzopyran?3?carboxaldehyde as a versatile starting material.
Abdel?Rahman A H, et al.
Heteroatom Chem., 16(1), 20-27 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service