N32601
Norcamphor
98%
Synonym(s):
2-Norbornanone, Bicyclo[2.2.1]heptan-2-one
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H10O
CAS Number:
Molecular Weight:
110.15
Beilstein:
1209657
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
168-172 °C (lit.)
mp
93-96 °C (lit.)
SMILES string
O=C1C[C@@H]2CC[C@H]1C2
InChI
1S/C7H10O/c8-7-4-5-1-2-6(7)3-5/h5-6H,1-4H2/t5-,6+/m1/s1
InChI key
KPMKEVXVVHNIEY-RITPCOANSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marko D Mihovilovic et al.
ChemSusChem, 1(1-2), 143-148 (2008-07-09)
Recombinant Escherichia coli cells expressing monooxygenases of different bacterial origin were evaluated in microbial Baeyer-Villiger oxidations of racemic fused ketones. During the enzymatic oxidation process, both the "normal" lactone generated by migration of the more-substituted carbon atom and/or the "abnormal"
J Contzen et al.
Biochemistry, 37(13), 4317-4324 (1998-04-29)
Step-scan time-resolved Fourier transform infrared spectroscopy with a time resolution of 5 micros was applied to the carbon monoxide complex of cytochrome P-450cam (CYP101) to study the bimolecular ligand-rebinding process after flash photolysis. Spectral changes in the CO ligand stretch
M B Bass et al.
Proteins, 13(1), 26-37 (1992-05-01)
While cytochrome P-450cam catalyzes the hydroxylation of camphor to 5-exo-hydroxycamphor with 100% stereospecificity, norcamphor is hydroxylated by this enzyme yielding 45% 5-exo-, 47% 6-exo-, and 8% 3-exo-hydroxynorcamphor (Atkins, W.M., Sligar, S.G., J. Am. Chem. Soc. 109:3754-3760, 1987). The present study
J R Collins et al.
The Journal of biological chemistry, 263(7), 3164-3170 (1988-03-05)
The hydroxylations of d-camphor, norcamphor, pericyclocamphanone, and 5,5-difluorocamphor by cytochrome P-450cam have been examined using theoretical methods to identify and characterize properties which determine product specificity. Experimental results indicate that each molecule is hydroxylated with quite different regio-specificity when metabolized
Sergio Abbate et al.
The journal of physical chemistry. A, 113(42), 11390-11405 (2009-09-30)
The absorption spectra and vibrational circular dichroism (VCD) spectra in the mid-IR range 1600-950 cm(-1) of 10 camphor-related compounds have been recorded and compared to DFT calculated spectra at the B3PW91/TZ2P level and have been examined together with the corresponding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service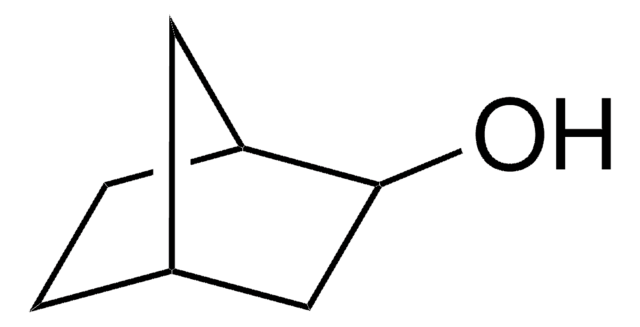





![Bicyclo[3.3.1]nonan-9-one ≥98%](/deepweb/assets/sigmaaldrich/product/structures/270/852/60661ded-13fb-4fc7-af36-a381880070a5/640/60661ded-13fb-4fc7-af36-a381880070a5.png)

![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)
