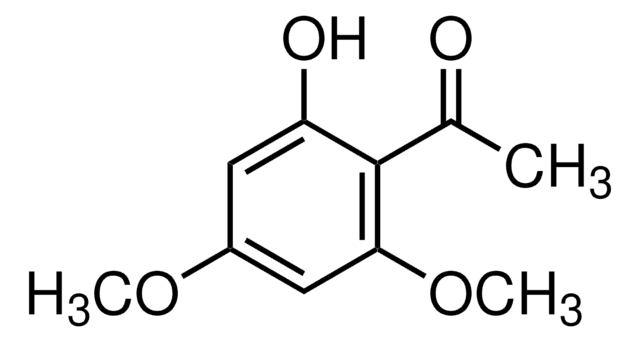
H35803
2′-Hydroxy-4′-methoxyacetophenone
99%
Synonym(s):
Paeonol, Resacetophenone 4-O-methyl ether
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H3(OCH3)COCH3
CAS Number:
Molecular Weight:
166.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
48-50 °C (lit.)
SMILES string
O=C(C)C1=CC=C(OC)C=C1O
InChI
1S/C9H10O3/c1-6(10)8-4-3-7(12-2)5-9(8)11/h3-5,11H,1-2H3
InChI key
UILPJVPSNHJFIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yue-Qin Wang et al.
Biological & pharmaceutical bulletin, 35(5), 767-772 (2012-06-13)
Atherosclerosis is a chronic inflammatory disease characterized by increased expression of adhesion molecules, which contribute to monocytes adhesion to vascular endothelial cells (VECs). Paeonol, an active compound isolated from cortex Moutan, has been shown to have therapeutic effects on atherosclerotic
Rui-guang Wu et al.
International journal of pharmaceutics, 438(1-2), 91-97 (2012-09-18)
Thermotropic phase behavior of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) liposomes containing 5 mol% cholesterol, or 5 mol% stigmasterol, or 5 mol% paeonol have been investigated by differential scanning calorimetry (DSC) and synchrotron X-ray diffraction (XRD) techniques, to investigate the competitive molecular interaction among
Ji-Yong Liu et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 47(2), 244-249 (2012-04-19)
Investigation of the pharmacokinetics of paeonol microemulsion, microemulsion-based gels and marketed paeonol ointments by the skin-blood synchronous microdialysis coupled with LC/MS is reported in this study. The microdialysis systems were established by linear probes and concentric circles probes. In vivo
Qiuling Wang et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 37(7), 920-924 (2012-07-17)
To study on the effect of different processing methods on the contents of seven major constituents in wild and cultivated Paeonia lactiflora, gallic acid, catechin, albiflorin, paeoniflorin, pentagalloylglucose, benzoic acid and paeonol, in order to provide reference basis for different
Liqin Ding et al.
Xenobiotica; the fate of foreign compounds in biological systems, 42(12), 1206-1212 (2012-06-12)
Paeonol, a major component of Paeonia suffruticosa Andrews, is used in clinical situations in China as a natural anti-inflammatory agent. The aim of the present study is to investigate the metabolism of paeonol in humans. Six metabolites were isolated from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service