All Photos(1)
About This Item
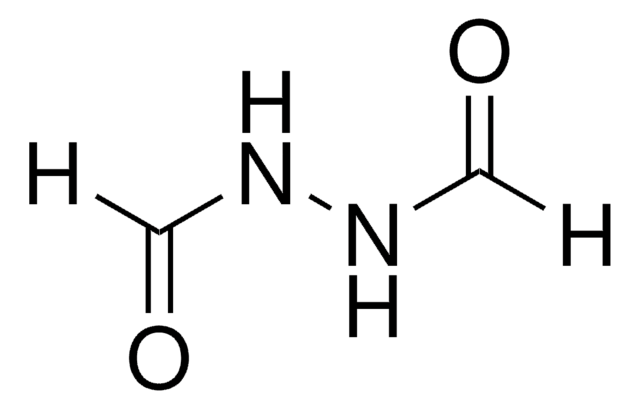
Linear Formula:
CH3CONHNHCOCH3
CAS Number:
Molecular Weight:
116.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
209 °C/15 mmHg (lit.)
mp
138-140 °C (lit.)
SMILES string
CC(=O)NNC(C)=O
InChI
1S/C4H8N2O2/c1-3(7)5-6-4(2)8/h1-2H3,(H,5,7)(H,6,8)
InChI key
ZLHNYIHIHQEHJQ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E B Bhalerao et al.
Indian journal of physiology and pharmacology, 29(2), 83-88 (1985-04-01)
Patients suffering from pulmonary tuberculosis were investigated for the levels of isoniazid (INH) and its metabolites viz. acetyl-INH, mono and diacetyl hydrazines and ammonia. It was observed that 50% of the patients are slow inactivators of INH and almost all
Ramachandran Azhakar et al.
Dalton transactions (Cambridge, England : 2003), 41(5), 1529-1533 (2011-12-14)
The reaction of N-heterocyclic silylene (NHSi) L [L = CH{(C[double bond, length as m-dash]CH(2))(CMe)(2,6-iPr(2)C(6)H(3)N)(2)}Si] with benzoylhydrazine, 1,2-dicarbethoxyhydrazine, 1,2-diacetylhydrazine and 1,2-bis(tert-butoxycarbonyl)hydrazine in 1 : 1 molar ratio resulted in compounds 1-4 with an almost quantitative yield and five coordinate silicon atoms.
J A Timbrell et al.
Human toxicology, 3(6), 485-495 (1984-12-01)
The urinary metabolite profile of isoniazid has been studied in patients receiving the drug as therapy for tuberculosis and the profile in patients suffering liver damage due to isoniazid compared with that in control patients. There were no consistent differences
W von Sassen et al.
Journal of chromatography, 338(1), 113-122 (1985-02-27)
A high-performance liquid chromatographic assay for the determination of isoniazid, acetylisoniazid, acetylhydrazine and diacetylhydrazine (plasma and urine) was developed. The m-chlorobenzoyl derivatives of isoniazid, acetylhydrazine and the internal standard propionylhydrazine were prepared, separated on a RP-18 column and detected at
Qiqi Zhao et al.
Journal of agricultural and food chemistry, 56(13), 5254-5259 (2008-06-11)
A series of novel N-substituted phenoxysulfenyl- N'- tert-butyl- N, N'-diacylhydrazines were designed and synthesized as insect growth regulators via the key intermediates N-chlorosulfenyl- N'- tert-butyl- N, N'-diacylhydrazines. Compared to the parent compounds, these N-substituted phenoxysulfenyl derivatives displayed better solubility and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service