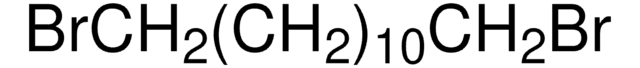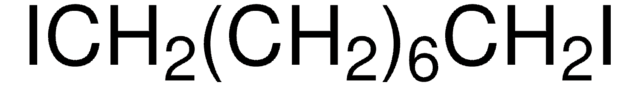D39800
1,10-Dibromodecane
97%
Synonym(s):
Decamethylene dibromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Br(CH2)10Br
CAS Number:
Molecular Weight:
300.07
Beilstein:
506156
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
refractive index
n20/D 1.4912 (lit.)
bp
160 °C/15 mmHg (lit.)
mp
25-27 °C (lit.)
density
1.335 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCCCBr
InChI
1S/C10H20Br2/c11-9-7-5-3-1-2-4-6-8-10-12/h1-10H2
InChI key
GTQHJCOHNAFHRE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,10-Dibromodecane, also known as Decamethylene dibromide, is an aromatic and aliphatic monomer, often utilized in the polymerization reaction to synthesize high molecular weight polysulfides through polycondensation.
Application
1,10-Dibromodecane is used:
- as a reactant in the Wurtz-type reaction during the synthesis of polyethylene
- Construction of pillar[4]arene[1]quinone-1,10-dibromodecane pseudorotaxanes in solution and in the solid state.: This research highlights the application of 1,10-Dibromodecane in constructing complex molecular structures known as pseudorotaxanes. The study focuses on the synthesis and stabilization of these structures both in solution and solid states, providing a foundational technique for developing advanced materials in chemical engineering, pharmaceutical synthesis, and high-performance materials within industrial chemical manufacturing sectors. This breakthrough offers potential pathways for new drug delivery systems and smart materials based on the unique properties of these supramolecular assemblies (Sheng et al., 2020).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of polysulfides containing s-triazine rings from 6-substituted amino-1, 3, 5-triazine-2, 4-dithiols and 1, 10-dibromodecane
Yoshiyuki O, et al.
Macromolecular Rapid Communications, 20, 294-298 (1999)
Stefania Aivali et al.
The journal of physical chemistry. B, 124(24), 5079-5090 (2020-05-28)
Conjugation-break flexible spacers in-between π-conjugated segments were utilized herein toward processable perylene diimide (PDI)-based polymers. Aromatic-aliphatic PDI-based polymers were developed via the two-phase polyetherification of a phenol-difunctional PDI monomer and aliphatic dibromides. These polyethers showed excellent solubility and film-forming ability
Ruili Zhang et al.
Advanced healthcare materials, 9(14), e2000394-e2000394 (2020-06-17)
The complexity of biological systems poses a great challenge in the development of nanotheranostic agents with enhanced therapeutic efficacies. To systematically overcome a series of barriers during in vivo administration and achieve optimal antitumor activity, nanotheranostic agents that can self-adaptively
Chemical structures, properties, and applications of selected crude oil-based and bio-based polymers
Piotr K, et al.
Polymers, 14, 5551-5551 (2022)
Design and Synthesis of Imidazolium-Mediated Tro?ger?s Base-Containing Ionene Polymers for Advanced CO2 Separation Membranes
Irshad k, et al.
ACS Omega, 4, 3439-3448 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service