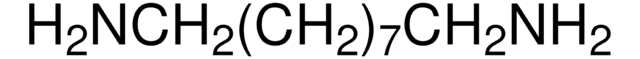D17408
1,7-Diaminoheptane
98%
Synonym(s):
1,7-Heptanediamine, Heptamethylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2(CH2)7NH2
CAS Number:
Molecular Weight:
130.23
Beilstein:
1734159
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
223-225 °C (lit.)
mp
26-29 °C (lit.)
SMILES string
NCCCCCCCN
InChI
1S/C7H18N2/c8-6-4-2-1-3-5-7-9/h1-9H2
InChI key
PWSKHLMYTZNYKO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,7-Diaminoheptane, also known as heptamethylenediamine, is an organic compound with two amine groups at opposite ends of a seven-carbon chain. It is widely used in organic synthesis as a building block in the production of polyamides and other polymers.
Application
- Novel Hydrogels for Biomedical Applications: Research on the synthesis and characterization of pH-responsive aminated alginate derivatives using 1,7-Diaminoheptane, targeting advancements in tissue engineering and drug delivery systems. This study showcases the utility of 1,7-Diaminoheptane in developing hydrogels that respond to pH changes, potentially enhancing cell growth and targeted drug release (Khodayar et al., 2023).
- Advanced Materials in Photoluminescence: Investigation into the properties of 1,7-Diaminoheptane in enhancing broadband photoluminescence in hybrid perovskites for optoelectronic applications. This research highlights its potential in developing new materials for efficient light emission technologies (Deng et al., 2020).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sheila L Flack et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 878(27), 2635-2642 (2010-02-24)
1,6-Hexamethylene diisocyanate (HDI) is extensively used in the automotive repair industry and is a commonly reported cause of occupational asthma in industrialized populations. However, the exact pathological mechanism remains uncertain. Characterization and quantification of biomarkers resulting from HDI exposure can
Chun-Yang Sun et al.
Theranostics, 8(11), 2939-2953 (2018-06-14)
The simple integration of chemotherapeutic drugs and photosensitizers (PSs) into the same nanocarriers only achieves a combination of chemo-photodynamic therapy but may not confer synergistic effects. The boosted intracellular release of chemotherapeutic drugs during the photodynamic therapy (PDT) process is
G Taibi et al.
Journal of chromatography, 614(1), 153-158 (1993-04-21)
A rapid reversed-phase high-performance liquid chromatographic method, using pre-column derivatization with benzoyl chloride and ultraviolet detection at 254 nm, was developed for the simultaneous measurement of polyamines and their monoacetyl derivatives. Calibration curves were linear for concentrations from 1.25 to
Irena Kralj Cigić et al.
Foods (Basel, Switzerland), 9(5) (2020-05-07)
Sprouts and microgreens are a rich source of various bioactive compounds. Seeds of lentil, fenugreek, alfalfa, and daikon radish seeds were germinated and the contents of the polyamines agmatine (AGM), putrescine (PUT), cadaverine (CAD), spermidine (SPD), and spermine (SPM) in
Y B Lee et al.
Bioorganic & medicinal chemistry, 6(3), 253-270 (1998-05-06)
Deoxyhypusine synthase catalyzes the first step in the posttranslational biosynthesis of the unusual amino acid hypusine [N epsilon-(4-amino-2-hydroxybutyl)lysine] in eukaryotic translation initiation factor 5A (eIF-5A). eIF-5A and its single hypusine residue are essential for cell proliferation. Two series of 1,7-diaminoheptane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service