All Photos(1)
About This Item
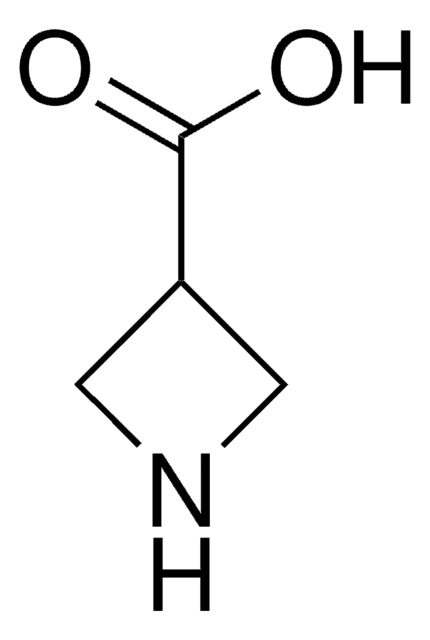
Empirical Formula (Hill Notation):
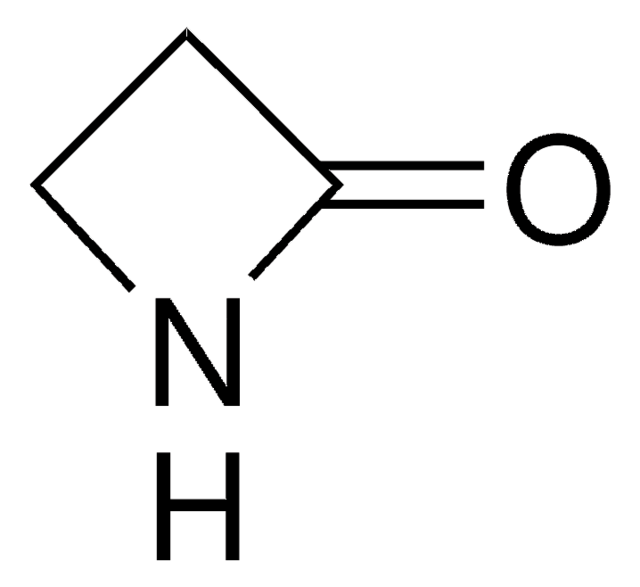
C4H7NO2
Molecular Weight:
101.10
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
Recommended Products
form
solid
SMILES string
OC(=O)C1CCN1
InChI
1S/C4H7NO2/c6-4(7)3-1-2-5-3/h3,5H,1-2H2,(H,6,7)
InChI key
IADUEWIQBXOCDZ-UHFFFAOYSA-N
Other Notes
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jong Hum Kim et al.
Proceedings of the National Academy of Sciences of the United States of America, 114(46), E10009-E10017 (2017-11-01)
Ubiquitin E3 ligases are crucial for eliminating misfolded proteins before they form cytotoxic aggregates that threaten cell fitness and survival. However, it remains unclear how emerging misfolded proteins in the cytoplasm can be selectively recognized and eliminated by E3 ligases
Jane A Dalley et al.
Molecular biology of the cell, 19(7), 2876-2884 (2008-05-02)
Targeting of proteins to the endoplasmic reticulum (ER) occurs cotranslationally necessitating the interaction of the signal recognition particle (SRP) and the translocon with the ribosome. Biochemical and structural studies implicate ribosomal protein Rpl25p as a major ribosome interaction site for
Fu Yan et al.
ACS chemical biology, 14(1), 99-105 (2018-12-13)
S-Adenosyl-l-methionine (SAM)-dependent methyltransferases are intensely studied because they play important roles in the methylation of biomolecules in all domains of life. In this study, we describe that the methyltransferase VioH from Cysotobacter violaceus catalyzes a so far unknown cyclization of
Martin Klapper et al.
Cell chemical biology, 25(6), 659-665 (2018-04-03)
Chemical and biochemical analyses of one of the most basic nonribosomal peptide synthetases (NRPS) from a Pseudomonas fluorescens strain revealed its striking plasticity. Determination of the potential substrate scope enabled us to anticipate novel secondary metabolites that could subsequently be
Tatsuki Sato et al.
BioMed research international, 2020, 7245782-7245782 (2020-12-05)
Candida albicans undergoes a yeast-to-hyphal transition that has been recognized as a virulence property as well as a turning point leading to biofilm formation associated with candidiasis. It is known that yeast-to-hyphal transition is induced under complex environmental conditions including
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![tert-Butyl 2-oxo-1-oxa-8-azaspiro[4.5]decane-8-carboxylate](/deepweb/assets/sigmaaldrich/product/structures/108/251/f0893bce-f9a2-48e1-bbea-68aaaa08e2e7/640/f0893bce-f9a2-48e1-bbea-68aaaa08e2e7.png)

