C112208
Cyclopentanol
99%
Synonym(s):
1-Cyclopentanol, Cyclopentyl alcohol, Hydroxycyclopentane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
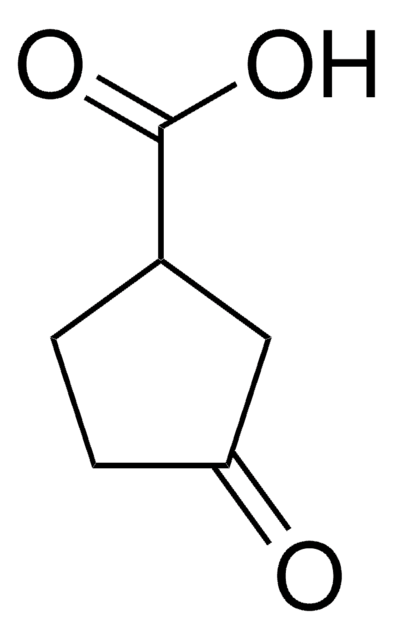
Linear Formula:
C5H9OH
CAS Number:
Molecular Weight:
86.13
Beilstein:
1900556
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.453 (lit.)
bp
139-140 °C (lit.)
mp
−19 °C (lit.)
density
0.948 g/mL at 20 °C
0.949 g/mL at 25 °C (lit.)
SMILES string
OC1CCCC1
InChI
1S/C5H10O/c6-5-3-1-2-4-5/h5-6H,1-4H2
InChI key
XCIXKGXIYUWCLL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclopentanol can be used as:
- An alkylating agent in the preparation of alkylated aromatic compounds using Fe3+-montmorillonite catalyst via Friedel–Crafts alkylation reaction.
- A reactant in the acylation of alcohols with an acid anhydride or acid chloride.
- A substrate in the synthesis of high-density polycyclic aviation fuel by the Guerbet reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
123.8 °F - closed cup
Flash Point(C)
51 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
May Xiao-Wu Jiang et al.
The Journal of organic chemistry, 70(7), 2824-2827 (2005-03-25)
[reaction: see text] Enzymatic resolution of Boc-protected 4-aminocyclopenten-1-ol 4c gave both enantiomers 5c and 6c in high ee. Boc removal and separate condensation with chloropyrazolopyrimidine 18 provided elaborated 1,4-aminocyclopentenol derivatives 20 and 26, respectively. Separate treatment of 20 and 26
Marie Bøjstrup et al.
Organic & biomolecular chemistry, 5(19), 3164-3171 (2007-09-20)
Bicyclic cyclopentane lactones, prepared from bromodeoxyaldonolactones, were transformed into aminocyclopentanols with an Overman rearrangement as the key step. Two of the compounds prepared, 7 and 19, were found to be good inhibitors of jack bean alpha-mannosidase and beta-D-N-acetylglucosaminidase, respectively.
Highly efficient acylation of alcohols, amines and thiols under solvent-free and catalyst-free conditions.
Ranu BC, et al.
Green Chemistry, 5(1), 44-46 (2003)
S Dallet et al.
Biochimica et biophysica acta, 1294(1), 15-24 (1996-05-02)
A comparison between the pressure effects on the catalysis of Thermoanaerobium brockii alcohol dehydrogenase (TBADH: a thermostable tetrameric enzyme) and yeast alcohol dehydrogenase (YADH: a mesostable tetrameric enzyme) revealed a different behaviour. YADH activity is continuously inhibited by an increase
Synthesis of high density aviation fuel with cyclopentanol derived from lignocellulose.
Sheng X, et al.
Scientific Reports, 5(1), 9565-9565 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service