B97208
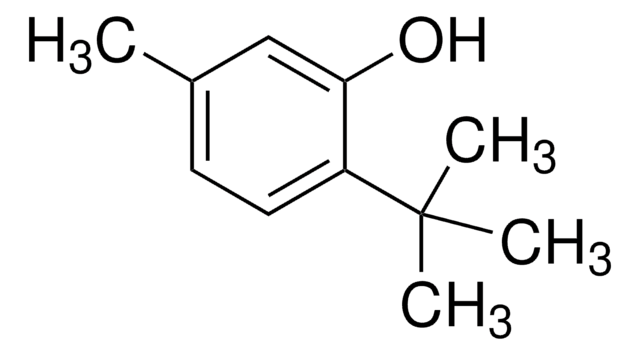
2-tert-Butyl-4-methylphenol
99%
Synonym(s):
2-tert-Butyl-p-cresol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CC6H3(CH3)OH
CAS Number:
Molecular Weight:
164.24
Beilstein:
1817645
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
bp
244 °C (lit.)
mp
50-52 °C (lit.)
SMILES string
Cc1ccc(O)c(c1)C(C)(C)C
InChI
1S/C11H16O/c1-8-5-6-10(12)9(7-8)11(2,3)4/h5-7,12H,1-4H3
InChI key
IKEHOXWJQXIQAG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
212.0 °F - closed cup
Flash Point(C)
100 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Hirose et al.
Cancer research, 51(3), 824-827 (1991-02-01)
The combined effects of low doses of promoters or carcinogens on two-stage forestomach carcinogenesis were examined in rats pretreated with N-methyl-N'-nitro-N-nitrosoguanidine. Groups of 15 rats were given a single 150 mg/kg body weight intragastric dose of N-methyl-N'-nitro-N-nitrosoguanidine. Starting 1 week
P Lambelet et al.
Skin pharmacology : the official journal of the Skin Pharmacology Society, 1(2), 115-121 (1988-01-01)
Radical reactions of anthralin and its metabolites with skin have been studied by ESR spectroscopy. The influence of compounds which are known to suppress inflammation are described. The ESR spectra recorded during the reaction of anthralin with skin were essentially
Y Kurata et al.
Japanese journal of cancer research : Gann, 81(8), 754-759 (1990-08-01)
The urinary bladder tumor-promoting potentials of the phenolic antioxidants, 2-tert-butyl-4-methylphenol (TBMP), propylparaben, catechol, resorcinol and hydroquinone, which are structurally related to butylated hydroxyanisole (BHA), were investigated in 170 male F344 rats. The animals were initially given 0.05% N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) as
M A Shibata et al.
Carcinogenesis, 11(3), 425-429 (1990-03-01)
The effects of 8 weeks of oral administration of five different phenolic antioxidants, e.g. catechol (CC), resorcinol, hydroquinone (HQ), 2-tert-butyl-4-methylphenol (TBMP) and propylparabene (PP), on forestomach and glandular stomach epithelium of male F344 rats were evaluated using a combined immunohistochemical
Shudong Zhang et al.
AAPS PharmSciTech, 20(2), 75-75 (2019-01-12)
Drugs with pH-dependent solubility that have poor water solubility can be identified in the drug discovery pipeline. Some of them have poor oral absorption, which can result in insufficient efficacy. Micro-environmental pH-modifying solid dispersion (micro pHm SD) is a promising
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service