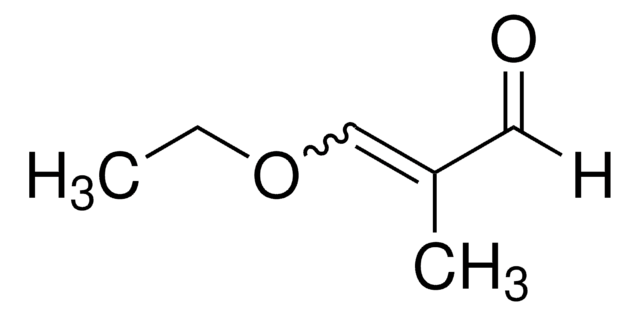
A24001
Acrolein diethyl acetal
96%
Synonym(s):
3,3-Diethoxy-1-propene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH(OCH2CH3)2
CAS Number:
Molecular Weight:
130.18
Beilstein:
1701567
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.398 (lit.)
bp
125 °C (lit.)
density
0.854 g/mL at 25 °C (lit.)
SMILES string
CCOC(OCC)C=C
InChI
1S/C7H14O2/c1-4-7(8-5-2)9-6-3/h4,7H,1,5-6H2,2-3H3
InChI key
MCIPQLOKVXSHTD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Acrolein diethyl acetal is widely used to carry out chemoselective Heck arylation to synthesize either 3-arylpropanoate esters or cinnamaldehyde derivatives.
It can also be used as one of the precursors to synthesize natural products like (−)-(Z)-Deoxypukalide, (−)-Laulimalide, botryodiplodin, neolaulimalide and isolaulimalide.
It can also be used as one of the precursors to synthesize natural products like (−)-(Z)-Deoxypukalide, (−)-Laulimalide, botryodiplodin, neolaulimalide and isolaulimalide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
59.0 °F - closed cup
Flash Point(C)
15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An efficient palladium-catalyzed synthesis of cinnamaldehydes from acrolein diethyl acetal and aryl iodides and bromides.
Battistuzzi G, et al.
Organic Letters, 5(5), 777-780 (2003)
Stefano Parisotto et al.
Organic & biomolecular chemistry, 15(4), 884-893 (2017-01-04)
As part of our ongoing work on the synthesis of a new class of plant hormones named Strigolactones (SLs) and their analogues, we became interested in tracing bioactive molecules with red emitting BODIPY fluorophores in order to unravel signaling and
Mohammad Saidur Rhaman et al.
Plant & cell physiology, 61(5), 967-977 (2020-03-08)
Myrosinase (β-thioglucoside glucohydrolase, enzyme nomenclature, EC 3.2.1.147, TGG) is a highly abundant protein in Arabidopsis guard cells, of which TGG1 and TGG2 function redundantly in abscisic acid (ABA)- and methyl jasmonate-induced stomatal closure. Reactive carbonyl species (RCS) are α,β-unsaturated aldehydes
Synthesis of (?)-and (−)-botryodiplodin using stereoselective radical cyclizations of acyclic esters and acetals.
Nouguier R et al.
Tetrahedron Asymmetry, 14(19), 3005-3018 (2003)
Total Synthesis of Microtubule-Stabilizing Agent (-)-Laulimalide1.
Ghosh AK et al.
The Journal of Organic Chemistry, 66(26), 8973-8982 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service