86870
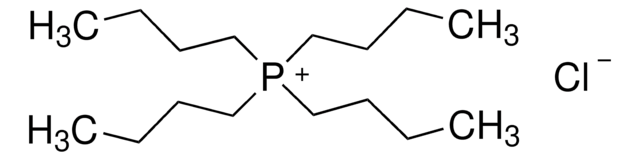
Tetrabutylammonium chloride
≥97.0% (NT)
Synonym(s):
N,N,N-tributyl-butanaminium chloride
About This Item
Recommended Products
Quality Level
Assay
≥97.0% (NT)
form
crystals
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤1% water
solubility
H2O: 20 mg/mL, clear, colorless
greener alternative category
, Aligned
SMILES string
[Cl-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.ClH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
InChI key
NHGXDBSUJJNIRV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Find details here.
Application
- 2-Amino-4H-chromene derivatives by the condensation reaction of aldehydes, malononitrile, and α, or β-naphthol.
- Methyl esters by esterification reaction of carboxylic acids with dimethyl carbonate in the presence of K2CO3.
TBACl can also be used with phosphorus pentoxide for greener deoxychlorination.
Process for Producing Halogenated Heteroaryl Compounds
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service