761494
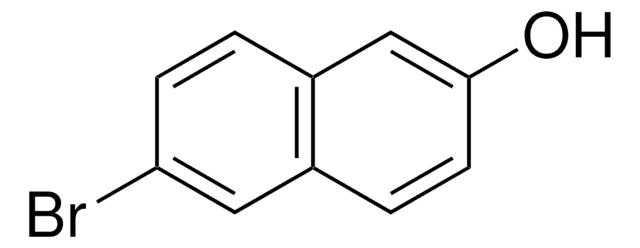
4-Bromo-1-naphthol
95%
Synonym(s):
1-Bromo-4-hydroxynaphthalene, 4-Bromo-1-naphthalenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H7BrO
CAS Number:
Molecular Weight:
223.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
128-133 °C
functional group
bromo
SMILES string
Oc1ccc(Br)c2ccccc12
InChI
1S/C10H7BrO/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6,12H
InChI key
OUNQUWORSXHSJN-UHFFFAOYSA-N
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
257.0 °F - closed cup
Flash Point(C)
125 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Marko Weimar et al.
The Journal of organic chemistry, 75(8), 2718-2721 (2010-03-23)
Starting from Dane's diene and methylcyclopentenedione, (+)-estrone is synthesized along the Quinkert-Dane route in 24% total yield. The key step is an enantioselective Diels-Alder reaction promoted by an amidinium catalyst as efficiently as by a traditional Ti-TADDOLate Lewis acid.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service