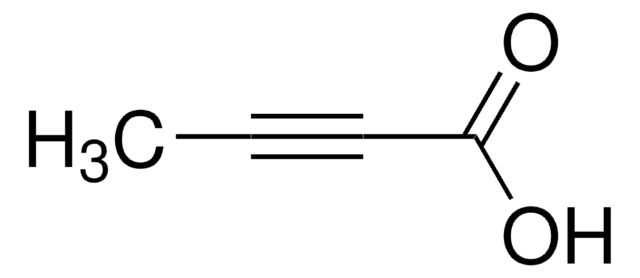738271
3-Butynoic acid
95%
Synonym(s):
2-Ethynylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H4O2
CAS Number:
Molecular Weight:
84.07
UNSPSC Code:
12352100
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
mp
70-80 °C
InChI
1S/C4H4O2/c1-2-3-4(5)6/h1H,3H2,(H,5,6)
InChI key
KKAHGSQLSTUDAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Some of the applications of 3-butynoic acid are:
- Synthesis of functionalized γ-butyrolactones and tetrahydrofurans via Conia-ene cyclizations.
- Synthesis of allenoates for [2+2] cycloadditions with alkenes.
- Phosphine-catalyzed synthesis of highly functionalized coumarins.
- Synthesis of various useful organotin reagents via radical hydrostannation of 3-butynoic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synergistic Diazo?OH Insertion/Conia?Ene Cascade Catalysis for the Stereoselective Synthesis of ??Butyrolactones and Tetrahydrofurans.
Hunter A C, et al.
Chemistry?A European Journal , 22(45), 16062-16065 (2016)
Catalytic Enantioselective Allenoate?Alkene [2+ 2] Cycloadditions.
Conner M L, et al.
Journal of the American Chemical Society, 137(10), 3482-3485 (2015)
Phosphine-catalyzed synthesis of highly functionalized coumarins.
Henry C E and Kwon O
Organic Letters, 9(16), 3069-3072 (2007)
Zhipeng Li et al.
The Analyst, 145(12), 4239-4244 (2020-05-22)
The aim of this study was to overcome the reported shortcomings of the glutathione (GSH) detection of rhodamine-based fluorescent probes, such as poor selectivity to thiol groups and reversible unstable covalent binding with the thiol groups. Here, we have developed
Ionogels, new materials arising from the confinement of ionic liquids within silica-derived networks.
Neouze M A, et al.
Chemistry of Materials, 18(17), 3931-3936 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service