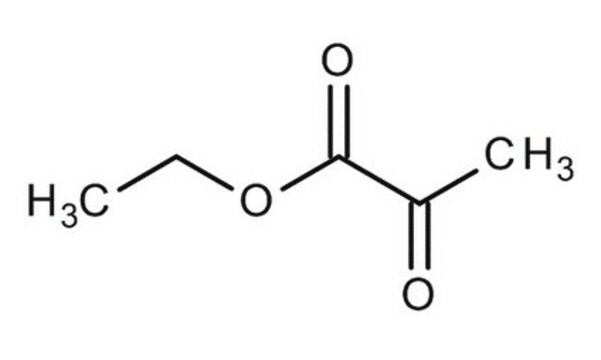
713309
Methyl 2-oxobutanoate
95%
Synonym(s):
2-Oxobutyric acid methyl ester, Methyl 2-ketobutyrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O3
CAS Number:
Molecular Weight:
116.12
Beilstein:
1749847
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0% (GC)
95%
form
liquid
impurities
≤1.0% water
functional group
ester
ketone
SMILES string
CCC(=O)C(=O)OC
InChI
1S/C5H8O3/c1-3-4(6)5(7)8-2/h3H2,1-2H3
InChI key
XPIWVCAMONZQCP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl 2-oxobutanoate can be used as a starting material:
- To synthesize indole derivatives via Fischer indolization of arylhydrazines using propylphosphonic anhydride.
- In the synthesis of (−)-picrotoxinin, a natural product and neurotoxic sesquiterpenoid.
- To prepare the core structure of phalarine, a natural product found in perennial grass.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
122.0 °F
Flash Point(C)
50 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of (−)-Picrotoxinin by Late-Stage Strong Bond Activation
Crossley SWM, et al.
Journal of the American Chemical Society, 142, 11376-11381 (2020)
Synthesis of the core structure of phalarine
Douki K, et al.
Organic & Biomolecular Chemistry, 17, 1727-1730 (2019)
A microwave-assisted, propylphosphonic anhydride (T3P) mediated one-pot Fischer indole synthesis
Desroses M, et al.
Tetrahedron Letters, 52, 4417-4420 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)