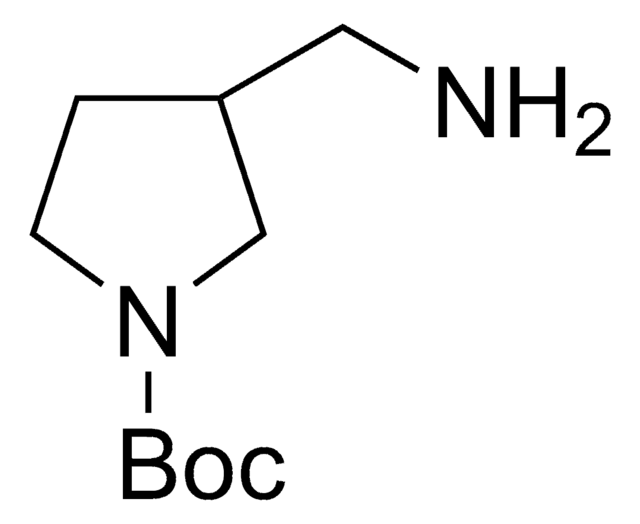
641472
1-Boc-4-(aminomethyl)piperidine
97%
Synonym(s):
1,1-Dimethylethyl 4-(aminomethyl)-1-piperidinecarboxylate, N-(tert-Butoxycarbonyl)-4-aminomethylpiperidine
About This Item
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.473 (lit.)
bp
237-238 °C (lit.)
density
1.013 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CC(C)(C)OC(=O)N1CCC(CN)CC1
InChI
1S/C11H22N2O2/c1-11(2,3)15-10(14)13-6-4-9(8-12)5-7-13/h9H,4-8,12H2,1-3H3
InChI key
KLKBCNDBOVRQIJ-UHFFFAOYSA-N
Related Categories
Application
- Kinesin spindle protein inhibitors with potential anticancer activity
- Orphan G-protein coupled receptor GPR119 agonist with antidiabetic potential
- Pim-1 inhibitors
- Aspartic acid protease inhibitors
Reactant involved in enantioselective synthesis of N-alkyl terminal aziridines
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service