All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H15N
CAS Number:
Molecular Weight:
161.24
Beilstein:
124508
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
61-65 °C (lit.)
functional group
phenyl
SMILES string
C1CC(CCN1)c2ccccc2
InChI
1S/C11H15N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-5,11-12H,6-9H2
InChI key
UTBULQCHEUWJNV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R A Glennon et al.
Journal of medicinal chemistry, 34(12), 3360-3365 (1991-12-01)
sigma receptors may represent an exciting new approach for the development of novel psychotherapeutic agents. Unfortunately, many of the commonly used sigma ligands lack selectivity (e.g., many bind at phencyclidine or dopamine receptors) or suffer from other serious drawbacks. Recently
Barbara Wenzel et al.
Bioorganic & medicinal chemistry letters, 22(6), 2163-2166 (2012-03-01)
This Letter describes the synthesis of two regioisomers of a new class of vesamicol analogs as possible ligands for imaging the vesicular acetylcholine transporter in future PET studies. The two pyrrolovesamicols (±)-6a and (±)-6b were synthesized by nucleophilic ring opening
Diane K Luci et al.
Bioorganic & medicinal chemistry letters, 17(23), 6489-6492 (2007-10-16)
Various 4-phenylpiperidine-benzoxazin-3-ones were synthesized and biologically evaluated as urotensin-II (U-II) receptor antagonists. Compound 12i was identified from in vitro evaluation as a low nanomolar antagonist against both rat and human U-II receptors. This compound showed in vivo efficacy in reversing
M G Russell et al.
Journal of medicinal chemistry, 35(11), 2025-2033 (1992-05-29)
This paper describes the synthesis of some conformationally restricted 4-phenylpiperidine analogues and their affinities for the guinea pig cerebellum sigma recognition site ([3H]-DTG) and the rat striatum dopamine D2 receptor ([3H]-(-)-sulpiride) in order to develop potent selective sigma ligands as
A G Ishkov et al.
Voprosy meditsinskoi khimii, 38(2), 25-28 (1992-03-01)
A rate of utilization of 4-phenyl piperidine and its 12 derivatives by brain monoamine oxidase (MAO) was studied as compared with typical neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The enzyme was isolated from P2 synaptosomal fraction of brain corpus striatum of Sprague-Dawley rats.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service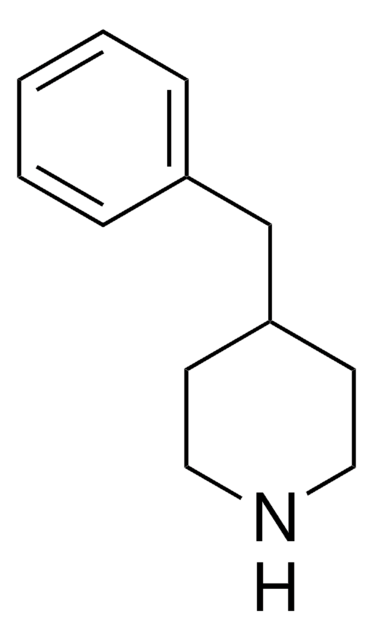





![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)


![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)

