578878
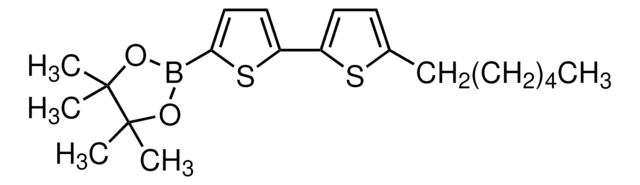
2,2′−Bithiophene-5-boronic acid pinacol ester
Synonym(s):
5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-2,2′-bithiophene
About This Item
Recommended Products
refractive index
n20/D 1.5900 (lit.)
Quality Level
mp
35.5-38.0 °C (average)
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(s2)-c3cccs3
InChI
1S/C14H17BO2S2/c1-13(2)14(3,4)17-15(16-13)12-8-7-11(19-12)10-6-5-9-18-10/h5-9H,1-4H3
InChI key
HPOQARMSOPOZMW-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Oligothiophenes are important organic electronic materials which can be produced using synthetic intermediates and Suzuki coupling.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service