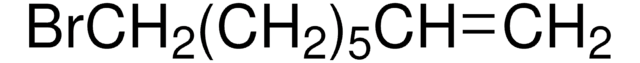All Photos(1)
About This Item
Linear Formula:
Br(CH2)8CH=CH2
CAS Number:
Molecular Weight:
219.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.4660 (lit.)
bp
50 °C/0.3 mmHg (lit.)
density
1.092 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCC=C
InChI
1S/C10H19Br/c1-2-3-4-5-6-7-8-9-10-11/h2H,1,3-10H2
InChI key
JVVPJOMYWVYPOF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
10-Bromo-1-decene can be synthesized by reacting 9-decen-1-ol with PBr3. The product undergoes reduction reaction with 2-propylbenzo[d][1,3,2]dioxaborole (PBD) and Bu3SnH under room temperature conditions to yield 1-decene.
Packaging
10-Bromo-1-decene may be used to synthesize:
- 4′-(9-decenyloxy) biphenyl-4-carboxylic acid methyl ester
- 4′-(9-decenyloxy)biphenyl-4-carboxylic acid
- 9-decenylmagnesium bromide, which was employed for the preparation of (R)-tridec-12-en-2-ol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
206.0 °F - closed cup
Flash Point(C)
96.67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The four-membered-ring chemical shift anomaly.
Lambert JB, et al.
The Journal of Organic Chemistry, 48(22), 3982-3985 (1983)
Julian Gebauer et al.
The Journal of organic chemistry, 71(5), 2021-2025 (2006-02-25)
A simple access to gamma,delta-unsaturated-beta-keto lactones is presented, allowing a rapid total synthesis of the naturally occurring 16-membered macrolide antibiotic (-)-A26771B via cross metathesis, asymmetric dihydroxylation, and lactonization as the key steps.
Novel ferroelectric and electroclinic organosiloxane liquid crystals.
Naciri J, et al.
Chemistry of Materials, 7(7), 1397-1402 (1995)
Room temperature ferroelectric terpolymers with large spontaneous polarization.
Naciri J, et al.
Macromolecules, 28(15), 5274-5279 (1995)
Radical initiation using borole derivatives.
Montgomery I, et al.
Tetrahedron Letters, 49(4), 628-630 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service