All Photos(2)
About This Item
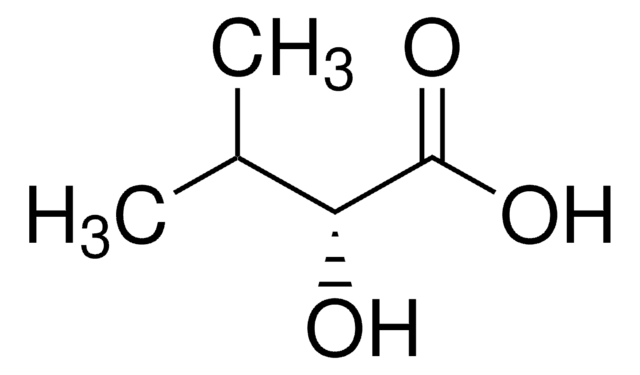
Empirical Formula (Hill Notation):
C4H8O3
CAS Number:
Molecular Weight:
104.10
Beilstein:
1720939
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (T)
form
solid
optical purity
enantiomeric ratio: ≥99:1 (GC)
mp
50-54 °C
functional group
carboxylic acid
hydroxyl
storage temp.
2-8°C
SMILES string
CC[C@@H](O)C(O)=O
InChI
1S/C4H8O3/c1-2-3(5)4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1
InChI key
AFENDNXGAFYKQO-GSVOUGTGSA-N
Other Notes
Chiral building block
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M N Romanelli et al.
Chirality, 8(8), 579-584 (1996-01-01)
The enantiomers of 3-alpha-tropyl 2-(phenylthio)butyrate (SM32, 1) were prepared by chiral synthesis and tested for analgesic, cognition-enhancing, and ACh-releasing properties. They show enantioselectivity in some of the tests, the eutomer being related in configuration to R-(+)-hyoscyamine.
K.J. Hale et al.
Tetrahedron Letters, 36, 6965-6965 (1995)
M N Romanelli et al.
Chirality, 8(3), 225-233 (1996-01-01)
The enantiomers of two alpha-tropanyl esters, SM21 (1) and PG9 (2), derived from (+)-R-hyoscyamine, that act by increasing the central cholinergic tone, were obtained by esterification after resolution of the corresponding racemic acids [(-)-S-1, (-)-R-2 and (+)-S-2] and by stereospecific
Ferhan Siddiqi et al.
Biochemistry, 44(25), 9013-9021 (2005-06-22)
Mandelate racemase (MR, EC 5.1.2.2) from Pseudomonas putida catalyzes the Mg(2+)-dependent 1,1-proton transfer that interconverts the enantiomers of mandelate. Crystal structures of MR reveal that the phenyl group of all ground-state ligands is located within a hydrophobic cavity, remote from
R Guerranti et al.
Biochimica et biophysica acta, 1568(1), 45-52 (2001-12-04)
Rat liver L-threonine dehydrogenase is a mitochondrial enzyme which transforms L-threonine either into aminoacetone or into acetyl-CoA. We show that it is inhibited by several fatty acids and their derivatives: short chain fatty acids, L-2-hydroxybutyrate and D-3-hydroxybutyrate, long chain fatty
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[(3R)-3-Hydroxybutyryl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/658/500/ff9570f8-a346-4077-9983-d0e67400bf47/640/ff9570f8-a346-4077-9983-d0e67400bf47.png)



![[(3R)-3-Hydroxyoctadecanoyl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/195/646/9c581614-9449-4107-a05e-7c573a907483/640/9c581614-9449-4107-a05e-7c573a907483.png)