All Photos(1)
About This Item
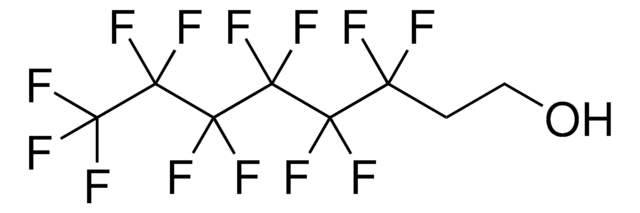
Linear Formula:
CF3CF2(CH2)3OH
CAS Number:
Molecular Weight:
178.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.33 (lit.)
bp
62-64 °C/35 mmHg (lit.)
density
1.35 g/mL at 25 °C (lit.)
functional group
fluoro
hydroxyl
SMILES string
OCCCC(F)(F)C(F)(F)F
InChI
1S/C5H7F5O/c6-4(7,2-1-3-11)5(8,9)10/h11H,1-3H2
InChI key
QROUUECTKRZFHF-UHFFFAOYSA-N
General description
4,4,5,5,5-Pentafluoro-1-pentanol, also known as pentafluoropentanol, is a perfluorous solvent.
Application
4,4,5,5,5-Pentafluoro-1-pentanol may be used in the preparation of chlorine-capped telechelic poly(methyl methacrylate)s.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
145.0 °F - closed cup
Flash Point(C)
62.8 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maria Carmelita Z Kasuya et al.
Biochemical and biophysical research communications, 316(3), 599-604 (2004-03-23)
Fluorous-tagged saccharide primers could be viable scaffolds for the synthesis of oligosaccharides. This research demonstrates that a fluorine-containing saccharide derivative could actually be taken up by the cell, the saccharide chain elongated by cellular enzymes, and the elongated product released
Yusuke Ogura et al.
Journal of the American Chemical Society, 138(15), 5012-5015 (2016-04-05)
Terminal-selective transesterification of chlorine-capped poly(methyl methacrylate)s (PMMA-Cl) with alcohols was developed as a modular approach to create telechelic and pinpoint-functionalized polymers. Being sterically less hindered and electronically activated, both the α-end ethyl ester and ω-end methyl ester of PMMA-Cl were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service