All Photos(1)
About This Item
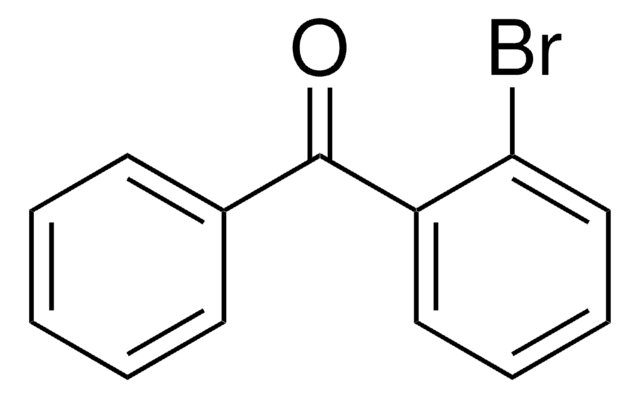
Linear Formula:
BrC6H4COC6H5
CAS Number:
Molecular Weight:
261.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
74.5-77.5 °C (lit.)
functional group
bromo
ketone
phenyl
SMILES string
Brc1cccc(c1)C(=O)c2ccccc2
InChI
1S/C13H9BrO/c14-12-8-4-7-11(9-12)13(15)10-5-2-1-3-6-10/h1-9H
InChI key
XNUMUNIJQMSNNN-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sonovoltammetric measurement of the rates of electrode processes with fast coupled homogeneous kinetics: Making macroelectrodes behave like microelectrodes.
Compton RG, et al.
Chemical Communications (Cambridge, England), 9, 1017-1018 (1996)
Mark F. Mechelke et al.
The Journal of organic chemistry, 64(13), 4821-4829 (2001-10-25)
The synthesis of a family of farnesol analogues, incorporating aromatic rings, has been achieved in high yields through the development of a regioselective coupling of allylic tetrahydropyranyl ethers with organometallic reagents. The allylic THP group is displaced readily by Grignard
Transition-metal catalyzed synthesis of ketoprofen.
Ramminger C, et al.
Journal of the Brazilian Chemical Society, 11(2), 105-111 (2000)
Similar or Totally Different: The Control of Conjugation Degree through Minor Structural Modifications, and Deep-Blue Aggregation-Induced Emission Luminogens for Non-Doped OLEDs.
Huang J, et al.
Advances in Functional Materials, 23(18), 2329-2337 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service