491047
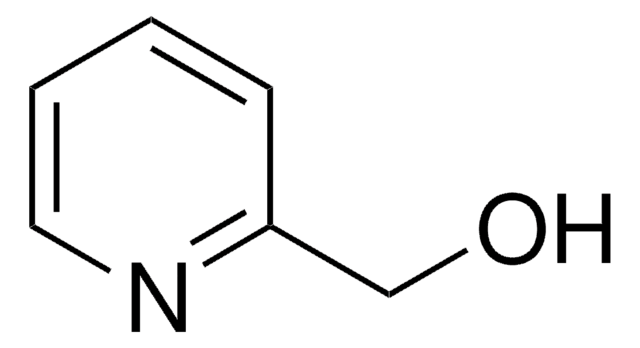
2-(Bromomethyl)pyridine hydrobromide
98%
Synonym(s):
(2-Pyridyl)methyl bromide hydrobromide, 2-Bromomethylpyridine monohydrobromide, 2-Picolyl bromide-hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H6BrN · HBr
CAS Number:
Molecular Weight:
252.93
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
149-152 °C (lit.)
functional group
bromo
SMILES string
Br[H].BrCc1ccccn1
InChI
1S/C6H6BrN.BrH/c7-5-6-3-1-2-4-8-6;/h1-4H,5H2;1H
InChI key
JQDNCGRNPYKRAO-UHFFFAOYSA-N
General description
2-(Bromomethyl)pyridine hydrobromide is a pyridine derivative. It participates in the synthesis of 7-nitrobenz-2-oxa-1,3-diazole (NBD) based colorimetric and fluorescence chemosensor.
Application
2-(Bromomethyl)pyridine hydrobromide may be used in the preparation of :
- 2-[1-(pyridin-2-ylmethyl)-1H-1,2,3-triazol-4-yl]pyridine
- 2-morpholin-4-yl-7-(pyridin-2-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 8-methyl-2-morpholin-4-yl-7-(pyridin-2-ylmethoxy)-4H-1,3-benzoxazin-4-one
- trans-4-(4-(bis(pyridin-2-ylmethyl)amino)styryl)benzonitrile
- 2-[N-(2-aminoethanethiol) methyl]-pyridine
- 2-[N-(ethylenediamino)-methyl]-pyridine
- 3-(carboxymethyl)-1-[(pyridin-2-yl)methyl]-3H-imidazol-1-ium trifluoroacetate
- 3-[(S)-1-carboxy-2-methylpropyl]-1-[(pyridin-2-yl)methyl]-3H-imidazol-1-ium trifluoroacetate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A stilbene-based fluoroionophore for copper ion sensing in both reduced and oxidized environments.
Zhu MQ, et al.
Talanta, 81(1), 678-683 (2010)
An NBD-based colorimetric and fluorescent chemosensor for Zn2+ and its use for detection of intracellular zinc ions.
Xu Z, et al.
Tetrahedron, 65(11), 2307-2312 (2009)
Synthesis and characterization of platinum (II) complexes from trifluoromethyl phenylenediamine, picoline and N-benzyl ethylenediamine derivatives.
Almeida MVD, et al.
Journal of the Brazilian Chemical Society, 17(7), 1266-1273 (2006)
Green Catalysts: Solid-Phase Peptide Carbene Ligands in Aqueous Transition-Metal Catalysis.
Worm-Leonhard K and Meldal M.
European Journal of Organic Chemistry, 2008(31), 5244-5253 (2008)
Saleh Ihmaid et al.
European journal of medicinal chemistry, 45(11), 4934-4946 (2010-08-31)
A number of new 2-amino-[5, 6, 7 and 8]-O-substituted 1,3-benzoxazines, and 2-amino 8-methyl-7-O-substituted-1,3-benzoxazines were synthesized. Thirty one new compounds were tested for their effect on collagen induced platelet aggregation and it was found that the most active compounds were 8-methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-4H-1,3-benzoxazin-4-one
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service