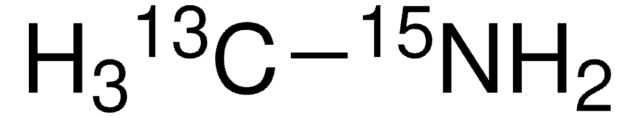All Photos(1)
About This Item
Linear Formula:
CH315NH2·HCl
CAS Number:
Molecular Weight:
68.51
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.12
Recommended Products
isotopic purity
98 atom % 15N
Quality Level
form
solid
mp
232-234 °C (lit.)
mass shift
M+1
SMILES string
Cl.C[15NH2]
InChI
1S/CH5N.ClH/c1-2;/h2H2,1H3;1H/i2+1;
InChI key
NQMRYBIKMRVZLB-CGOMOMTCSA-N
Related Categories
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas S Hofer et al.
Molecular bioSystems, 8(11), 2891-2900 (2012-08-01)
Molecular dynamics simulations have been performed to investigate the binding of tris(hydroxymethyl)-aminomethane to the surface of the core domain of the mouse cellular tumor antigen p53 employing the GROMOS and 53A6 force field parameter sets. A close investigation of the
April D Lewoczko et al.
Physical chemistry chemical physics : PCCP, 15(13), 4707-4714 (2013-02-21)
Using density functional theory calculations, we report on the adsorption of methylamine on gold and compare its adsorption to a selection of alkylamines, methanol and methanethiol. On the (111) surface, the amines, thiol and alcohol bind in the ontop site
Sheeza Khan et al.
Protein and peptide letters, 20(1), 61-70 (2012-06-08)
Kidney cells of animals including human and marine invertebrates contain high amount of the protein denaturant, urea. Methylamine osmolytes are generally believed to offset the harmful effects of urea on proteins in vitro and in vivo. In this study we
Frank Weinhold
Journal of computational chemistry, 33(30), 2440-2449 (2012-07-28)
We have developed a "Natural Bond Critical Point" (NBCP) module for the natural bond orbital (NBO) program that allows mutual analysis of NBO-based versus Bader-type quantum theory of atoms in molecules (QTAIM) topological descriptors of chemical bonding interactions. Conventional QTAIM
Nicolas Fleury-Brégeot et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(31), 9564-9570 (2012-07-07)
Ammoniomethyl trifluoroborates are very powerful reagents that can be used to access biologically relevant aryl- and heteroaryl-methylamine motifs via Suzuki-Miyaura cross-couplings. Until now, this method was limited to the production of tertiary and primary amines. The synthesis of a large
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service