47675
Formaldehyde diethyl acetal
absolute, over molecular sieve (H2O ≤0.01%), ≥99.0% (GC)
Synonym(s):
Diethoxymethane, Ethylal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
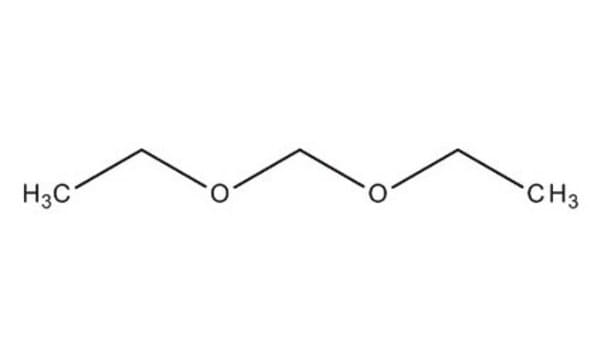
CH2(OC2H5)2
CAS Number:
Molecular Weight:
104.15
Beilstein:
1697253
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
3.6 (vs air)
vapor pressure
60 mmHg ( 25 °C)
grade
absolute
Assay
≥99.0% (GC)
form
liquid
autoignition temp.
346 °F
quality
over molecular sieve (H2O ≤0.01%)
refractive index
n20/D 1.373 (lit.)
n20/D 1.374
bp
87-88 °C (lit.)
density
0.831 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CCOCOCC
InChI
1S/C5H12O2/c1-3-6-5-7-4-2/h3-5H2,1-2H3
InChI key
KLKFAASOGCDTDT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Formaldehyde diethyl acetal (FDEA, diethoxymethane, DEM) is a diethyl acetal. It has been synthesized from ethanol and aqueous formaldehyde. It is a low boiling solvent useful for sodium hydride reactions, organolithium chemistry, copper-catalyzed conjugate additions and phase-transfer reactions. It is a potential alternate for tetrahydrofuran, dichloromethane, glyme and methylal. DEM is an ethoxymethylating agent, a formaldehyde equivalent and a carbonylation substrate. Its condensation with 2-propylresorcinol to form resorcin[n]arenes (n=4–7) has been investigated. The IR and Raman spectra and conformations of DEM have been studied. NMR spectroscopy has been used to study the aggregation behavior of organolithium compounds in diethoxymethane. Unimolecular metastable decomposition of DEM upon electron impact has been studied. Solubility of non-polar gases in DEM has been evaluated.
Application
Formaldehyde diethyl acetal may be used as a solvent in the esterification of carboxylic acids and in the O-alkylation of a variety of phenols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
23.0 °F - closed cup
Flash Point(C)
-5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solubility of non polar gases in formaldehyde diethyl acetal between-10 and 30?C, and 1 Atm partial pressure of gas.
Lizano LP, et al.
Journal of Solution Chemistry, 19(7), 721-728 (1990)
Metastable decompositions of gem-dialkoxyalkanes upon electron impact. III. Diethoxymethane (CH2 (OCH2CH3)2).
Tajima S, et al.
Rapid Communications in Mass Spectrometry, 14(14), 1195-1199 (2000)
Sc(OTf)3-catalyzed cyclocondensation of 2-propylresorcinol with diethoxymethane. Formation and fragmentation of resorcin [n] arenes.
Morikawa O, et al.
Tetrahedron Letters, 45(29), 5731-5734 (2004)
NMR spectroscopy of organolithium compounds, Part XVI: The aggregation behavior of butyllithium, phenyllithium, and lithium diisopropylamide in dimethoxy-and diethoxymethane.
Bergander K, et al.
Tetrahedron, 50(20), 5861-5868 (1994)
Use of Diethoxymethane as a Solvent for Phase-Transfer Esterification of Carboxylic Acids.
Coleman MT.
Synthetic Communications, 42(13), 1911-1913 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service