471070
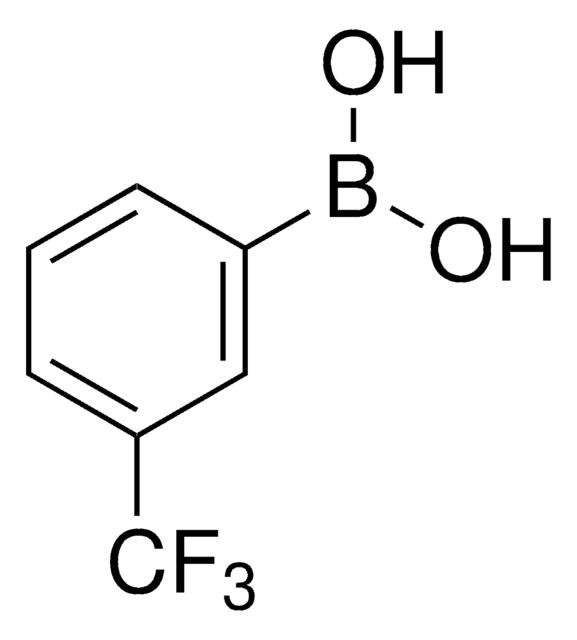
3,5-Bis(trifluoromethyl)phenylboronic acid
≥95%
Synonym(s):
3,5-Di(trifluoromethyl)benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CF3)2C6H3B(OH)2
CAS Number:
Molecular Weight:
257.93
Beilstein:
7379990
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
powder
mp
217-220 °C (lit.)
functional group
fluoro
SMILES string
OB(O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F
InChI
1S/C8H5BF6O2/c10-7(11,12)4-1-5(8(13,14)15)3-6(2-4)9(16)17/h1-3,16-17H
InChI key
BPTABBGLHGBJQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant involved in the synthesis of:
- Methylene-arylbutenones via carbonylative arylation of allenols
- 4-aminoquinoline analogs via Ullman / Suzuki / Negishi coupling
- Primary amino acid derivatives with anticonvulsant activity
- Alkyl arylcarbamates via Cu-catalyzed coupling with potassium cyanate
- Aryl-substituted succinimides and cyclic ketones by asymmetric conjugate addition
- Axially chiral dicarboxylic acids for asymmetric Mannich-type reactions
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F Adebodun et al.
Journal of cellular biochemistry, 40(2), 249-260 (1989-06-01)
31P Nuclear Magnetic Resonance (NMR) studies were performed on mono- and diisopropylphosphoryl derivatives of alpha-chymotrypsin, trypsin, and subtilisin. Questions addressed included the pKa of the active center Asp...His...Ser triad in both species. While the pKa in the diisopropylphosphoryl derivatives is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service