All Photos(2)
About This Item
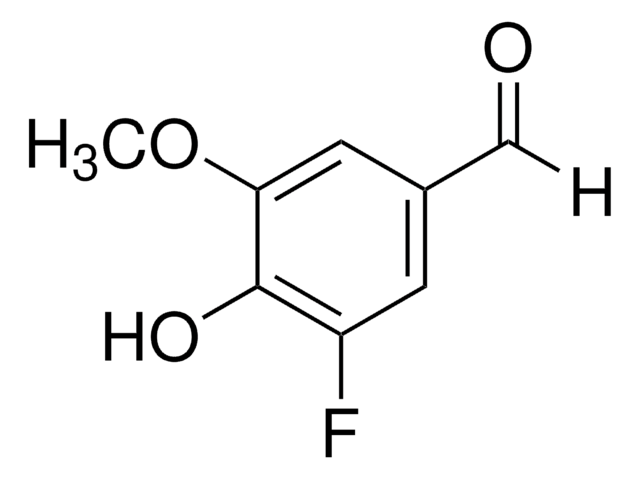
Linear Formula:
CH3OC6H2(OH)2CHO
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
131-134 °C (lit.)
functional group
aldehyde
SMILES string
COc1cc(C=O)cc(O)c1O
InChI
1S/C8H8O4/c1-12-7-3-5(4-9)2-6(10)8(7)11/h2-4,10-11H,1H3
InChI key
RRKMWVISRMWBAL-UHFFFAOYSA-N
General description
3,4-Dihydroxy-5-methoxybenzaldehyde can be obtained by reactting 5-iodovaniliin with sodium hydroxide and copper sulfate solution.
Application
3,4-Dihydroxy-5-methoxybenzaldehyde may be used for the preparation of 3,4-dihydroxy-6-methoxy-β-nitrostyrene and 5-hydroxyconiferyl alcohol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structure and synthesis of (?)-Wuweizisu C.
Schneiders GE and Stevenson R.
The Journal of Organic Chemistry, 46(41), 2969-2971 (1981)
MESCALINE ANALOGS. III. 2, 4, 6-TRIALKYL-AND 3, 4-DIHYDROXY-5-METHOXY-?-PHENETHYLAMINES.
Benington F, et al.
The Journal of Organic Chemistry, 20(9), 1292-1296 (1955)
Thomas Goujon et al.
Plant molecular biology, 51(6), 973-989 (2003-06-05)
A promoter-trap screen allowed us to identify an Arabidopsis line expressing GUS in the root vascular tissues. T-DNA border sequencing showed that the line was mutated in the caffeic acid O-methyltransferase 1 gene (AtOMT1) and therefore deficient in OMT1 activity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service