All Photos(1)
About This Item
Empirical Formula (Hill Notation):
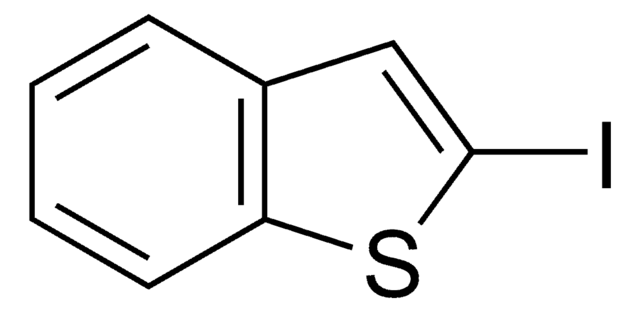
C5H5IS
CAS Number:
Molecular Weight:
224.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.626 (lit.)
bp
81-83 °C/10 mmHg (lit.)
density
1.852 g/mL at 25 °C (lit.)
functional group
iodo
storage temp.
2-8°C
SMILES string
Cc1ccc(I)s1
InChI
1S/C5H5IS/c1-4-2-3-5(6)7-4/h2-3H,1H3
InChI key
NAZNQEXKAPLVKC-UHFFFAOYSA-N
General description
2-Iodo-5-methylthiophene is a halogenated thiophene.
Application
2-Iodo-5-methylthiophene may be used for the preparation of:
- methylbis[2-(5-methyl)thienyl]borane
- dimethyl-terthienyl
- 5,5′-dimethy-2,2′-bithienyl
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
192.2 °F - closed cup
Flash Point(C)
89 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chromatographic and spectral characteristics of some polythienyls.
Sease JW and Zechmeister L.
Journal of the American Chemical Society, 69(2), 270-273 (1947)
Studies on the Electronic Absorption Spectra of 2, 2'-Bithienyl and Some of Its Derivatives. A Molecular Orbital Treatment.
Abu-Eittah RH and Al-Sugeir FA.
Bulletin of the Chemical Society of Japan, 58(7), 2126-2132 (1985)
Synthesis and Properties of Bis (2-heteroaryl) borane Derivatives.
Kohler T, et al.
European Journal of Inorganic Chemistry, 2002(11), 2942-2946 (2002)
Synthesis and pharmacological evaluation of (Z)-9-(heteroarylmethylene)-7-azatricyclo [4.3. 1.0 3, 7] decanes: Thiophene analogues as potent norepinephrine transporter inhibitors.
Zhou J, et al.
Bioorganic & Medicinal Chemistry Letters, 13(20), 3565-3569 (2003)
Alexander Koch et al.
Dalton transactions (Cambridge, England : 2003), 47(36), 12534-12539 (2018-05-05)
The reduction of 2-bromo- and 3-bromothiophene with calcium powder gives impure thienylcalcium complexes due to interference of various subsequent metalation and calcium-halogen exchange reactions as well as ether degradation. Therefore, calcium-iodine exchange succeeds via the reaction of trimethylsilylmethylcalcium halide with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)