446041
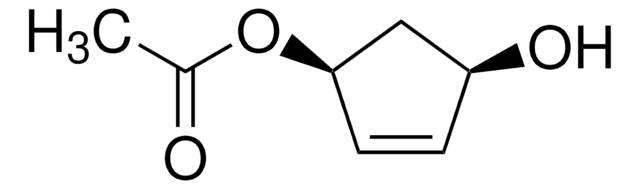
(1S,4R)-cis-4-Acetoxy-2-cyclopenten-1-ol
≥99%
Synonym(s):
(1R,3S)-(+)-cis-4-Cyclopentene-1,3-diol 1-acetate, (1R,3S)-4-Cyclopentene-1,3-diol 1-acetate, (1R,4S)-cis-4-Hydroxy-2-cyclopentenyl acetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H10O3
CAS Number:
Molecular Weight:
142.15
Beilstein:
4663992
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
solid
optical activity
[α]20/D +68°, c = 2.3 in chloroform
mp
49-51 °C (lit.)
SMILES string
CC(=O)O[C@@H]1C[C@H](O)C=C1
InChI
1S/C7H10O3/c1-5(8)10-7-3-2-6(9)4-7/h2-3,6-7,9H,4H2,1H3/t6-,7+/m1/s1
InChI key
IJDYOKVVRXZCFD-RQJHMYQMSA-N
Application
(1S,4R)-cis-4-Acetoxy-2-cyclopenten-1-ol can be used as:
- A building block for the synthesis of biologically significant carbocyclic nucleosides and prostaglandins.
- A starting material in the synthesis of azasugar analogs.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An improved preparation of highly enantiomerically enriched (R)-(+)-4-tert-butyldimethylsiloxy-2-cyclopenten-1-one
Myers AG, et al.
Tetrahedron Letters, 37(18), 3083-3086 (1996)
(4R)-(+)-tert-Butyldimethylsiloxy-2-Cyclopenten-1-one: 2-Cyclopenten-1-one, 4-[[(1, 1-dimethylethyl) dimethylsilyl] oxy]-,(R)-
Paquette LA,
Organic Syntheses, 73(18), 36-36 (1996)
Theil, F. et al.
Journal of the Chemical Society. Perkin Transactions 1, 255-255 (1996)
Variable and stereoselective synthesis of azasugar analogues by a ruthenium-catalyzed ring rearrangement
Voigtmann U and Blechert S
Organic Letters, 2(25), 3971-3974 (2000)
Synthesis of base-modified noraristeromycin derivatives and their inhibitory activity against human and Plasmodium falciparum recombinant S-adenosyl-L-homocysteine hydrolase
Kitade Y, et al.
Tetrahedron, 58(7), 1271-1277 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service