427055
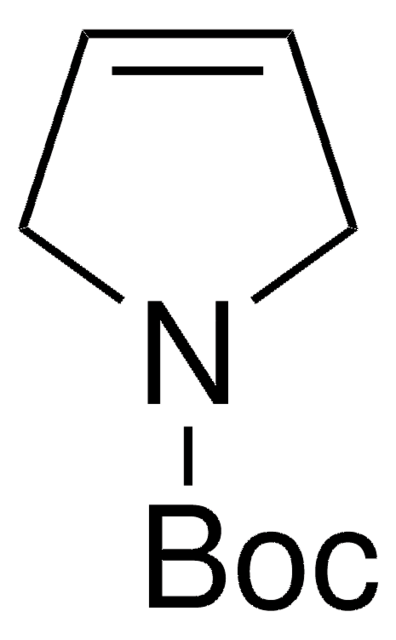
N-Boc-pyrrolidine
97%
Synonym(s):
tert-Butyl 1-pyrrolidinecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H17NO2
CAS Number:
Molecular Weight:
171.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.449 (lit.)
bp
80 °C/0.2 mmHg (lit.)
density
0.977 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)OC(=O)N1CCCC1
InChI
1S/C9H17NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H2,1-3H3
InChI key
LPQZERIRKRYGGM-UHFFFAOYSA-N
General description
N-Boc-pyrrolidine is an N-substituted pyrrolidine. It is reported that the reactivity of N-Boc-pyrrolidine towards C-H insertion reaction is 2000 times more than cyclohexane. It undergoes α-arylation in the presence of a palladium catalyst with high enantioselectivity.
Application
N-Boc-pyrrolidine may be used in the synthesis of the following:
- 2-aryl-N-boc-pyrrolidines
- scalemic 2-pyrrolidinylcuprates
- 2-alkenyl-N-Boc-pyrrolidines
- 1-deoxycastanospermine
- methylphenidate analogues
- (+)-elaeokanine A
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stereoselective synthesis of hydroxyindolizidines via sparteine-assisted deprotonation of N-Boc-pyrrolidine.
Majewski M, et al.
Tetrahedron Letters, 39(38), 6787-6790 (1998)
Enantioselective, palladium-catalyzed α-arylation of N-boc-pyrrolidine.
Campos KR, et al.
Journal of the American Chemical Society, 128(11), 3538-3539 (2006)
Copper mediated scalemic organolithium reagents in alkaloid syntheses.
3Dieter RK, et al.
Tetrahedron, 61(13), 3221-3230 (2005)
Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites.
Davies HML, et al.
Bioorganic & Medicinal Chemistry Letters, 14(7), 1799-1802 (2004)
Catalytic asymmetric C-H activation by methyl thiophen-3-yldiazoacetate applied to the synthesis of (+)-cetiedil.
Davies HML, et al.
Tetrahedron Letters, 43(28), 4981-4983 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service