40819
1-Benzyl-3-methylimidazolium tetrafluoroborate
≥97.0% (HPLC)
Synonym(s):
1-Methyl-3-benzylimidazolium tetrafluoroborate
About This Item
Recommended Products
Quality Level
Assay
≥97.0% (HPLC)
impurities
≤0.2% water
functional group
phenyl
SMILES string
F[B-](F)(F)F.C[n+]1ccn(Cc2ccccc2)c1
InChI
1S/C11H13N2.BF4/c1-12-7-8-13(10-12)9-11-5-3-2-4-6-11;2-1(3,4)5/h2-8,10H,9H2,1H3;/q+1;-1
InChI key
QULDEUUWXMCUFO-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Functionalized imidazolium cations with thioether, urea, or thiourea derivatized side chains act as metal-ligating moieties, whereas the PF6– anions provide the desired water immiscibility.
Functionalized imidazolium cations with thioether, urea, or thiourea derivatized side chains act as metal-ligating moieties, whereas the PF6– anions provide the desired water immiscibility.
Functionalized imidazolium cations with thioether, urea, or thiourea derivatized side chains act as metal-ligating moieties, whereas the PF6– anions provide the desired water immiscibility.
Functionalized imidazolium cations with thioether, urea, or thiourea derivatized side chains act as metal-ligating moieties, whereas the PF6– anions provide the desired water immiscibility.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service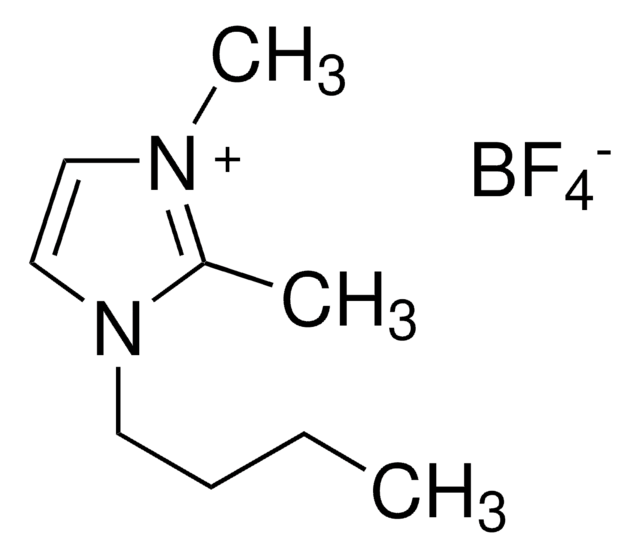







![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)

