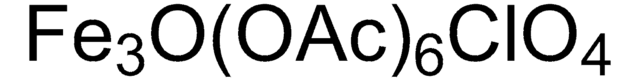399973
Cobalt(II) acetate
99.99% trace metals basis
Synonym(s):
Cobalt diacetate, Cobaltous acetate, Cobaltous diacetate
About This Item
Recommended Products
Quality Level
Assay
99.99% trace metals basis
form
crystals and lumps
solid
reaction suitability
core: cobalt
impurities
≤5% water
mp
298 °C (dec.) (lit.)
SMILES string
CC([O-])=O.[Co+2]
InChI
1S/2C2H4O2.Co/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
QAHREYKOYSIQPH-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A precursor to synthesize cobalt titanium oxide catalysts for the oxygen evolution reaction.
- A starting material to prepare polymer stabilized Co nanocatalyst for growing carbon nanofibers.
- A catalyst for direct amination of azoles under mild reaction conditions.
Cobalt(II) acetate can be:
- Used as a cobalt source in the synthesis of Lithium cobalt oxide (LiCoO2), which is a used as a cathode material in lithium-ion batteries.
- Used as a precursor to synthesize cobalt oxide nanoparticles via a simple direct thermal pyrolysis process. Co3O4 nanoparticles further used as a high-capacity anode materials in lithium-ion batteries.
- Used as an additive in the perovskite precursor solution to control the crystal growth and improve the performance of fully screen-printable hole-transport material (HTM)-free mesoporous perovskite solar cells (PSCs).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
The diversity of applications and nanostructured materials accessible using ultrasonic spray methods are highlighted in this article.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service