383449
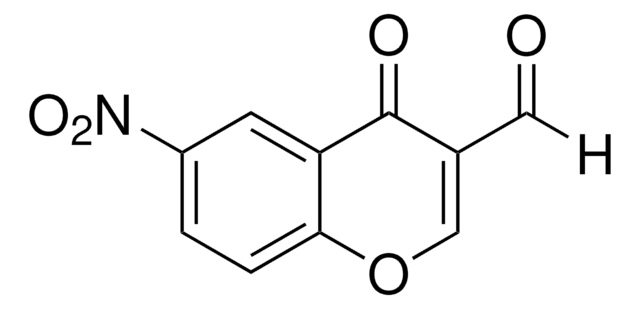
3-Formylchromone
97%
Synonym(s):
4-Oxo-4H-1-benzopyran-3-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H6O3
CAS Number:
Molecular Weight:
174.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
151-153 °C (lit.)
functional group
aldehyde
ketone
SMILES string
O=CC1=COc2ccccc2C1=O
InChI
1S/C10H6O3/c11-5-7-6-13-9-4-2-1-3-8(9)10(7)12/h1-6H
InChI key
FSMYWBQIMDSGQP-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
General description
Electrospray ionization mass spectrometry (ESI-MS) of protonated 3-formylchromone (3-FC) shows loss of H2 as a major fragmentation route to yield a ketene cation, which on reaction with water forms a protonated carboxylic acid. The invivo salubrious effects of 3-FC against nitrosodiethylamine (NDEA) mediated early hepatocellular carcinogenesis has been investigated. Synthesis and characterization of 3-FC and its derivatives has been reported.
Application
3-Formylchromone may be used in the following studies:
- Preparation of library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones, by three-component domino reactions with (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones and anilines under catalyst-free conditions.
- Facile and ecofriendly synthesis of new chromonyl chalcones.
- Synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrey S Plaskon et al.
The Journal of organic chemistry, 73(15), 6010-6013 (2008-07-03)
A facile and versatile procedure for the synthesis of 3-(2-hydroxybenzoyl)quinolines and 7H-chromeno[3,2-c]quinolin-7-ones was elaborated on the basis of TMSCl-mediated recyclization of 3-formylchromone with various anilines. Limitations and scope of this methodology were established, and a possible mechanism for the heterocyclizations
Muhammed Bilaal Ismail et al.
Nucleosides, nucleotides & nucleic acids, 38(12), 950-971 (2019-07-11)
Herein, we report the DNA interaction studies of rhenium(I) and -(V) compounds with Schiff base chelates encompassing biologically relevant moieties. More specifically, the DNA interaction capabilities of these rhenium complexes were probed using Gel Electrophoresis and Calf Thymus-DNA titrations monitored
Pitchaimani Prasanna et al.
Beilstein journal of organic chemistry, 10, 459-465 (2014-03-13)
The three-component domino reactions of (E)-3-(dimethylamino)-1-arylprop-2-en-1-ones, 3-formylchromone and anilines under catalyst-free conditions afforded a library of novel (E)-3-(2-arylcarbonyl-3-(arylamino)allyl)-4H-chromen-4-ones in good to excellent yields and in a diastereoselective transformation. This transformation generates one C-C and one C-N bond and presumably proceeds
Pedatsur Neta et al.
Rapid communications in mass spectrometry : RCM, 28(17), 1871-1882 (2014-08-05)
Electrospray ionization mass spectrometry (ESI-MS) of many protonated aldehydes shows loss of CO as a major fragmentation pathway. However, we find that certain aldehydes undergo loss of H2 followed by reaction with water in the collision cell. This complicates interpretation
Koichi Takao et al.
Bioorganic chemistry, 83, 432-437 (2018-11-15)
A series of eighteen pyrano[4,3-b][1]benzopyranone derivatives (1a-9b) were synthesized, and structure-activity relationships of their monoamine oxidase (MAO) A and B, acetylcholinesterase (AChE), and butyrylcholinesterase (BChE) inhibitory activities were evaluated. Most of the synthesized compounds exhibited weak inhibitory activity toward MAO-A
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service