All Photos(2)
About This Item
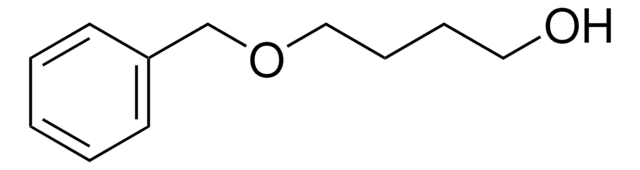
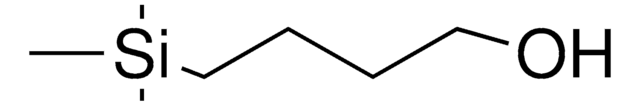
Linear Formula:
C6H5CH2O(CH2)3OH
CAS Number:
Molecular Weight:
166.22
Beilstein:
1864016
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
bp
111-114 °C/2 mmHg (lit.)
density
1.049 g/mL at 25 °C (lit.)
functional group
ether
hydroxyl
phenyl
SMILES string
OCCCOCc1ccccc1
InChI
1S/C10H14O2/c11-7-4-8-12-9-10-5-2-1-3-6-10/h1-3,5-6,11H,4,7-9H2
InChI key
FUCYABRIJPUVAT-UHFFFAOYSA-N
General description
3-Benzyloxy-1-propanol is an organic building block. It undergoes cleavage selectively at the C3-O position in the presence of ruthenium catalyst.
Application
3-Benzyloxy-1-propanol may be used as starting reagent in the total synthesis of (+)-cocaine. It may be used in the synthesis of a series of galactosyl phosphate diester derivatives of 9-β-D-arabinofuranosyladenine and 1-β-D-arabinofuranosylcytosine.
3-Benzyloxy-1-propanol may be used for the synthesis of 1-benzyloxy-3-iodopropane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cheng Chen et al.
Organic letters, 14(12), 2992-2995 (2012-06-06)
The ruthenium catalyzed selective sp(3) C-O cleavage with amide formation was reported in reactions of 3-alkoxy-1-propanol derivatives and amines. The cleavage only occurs at the C3-O position even with 3-benzyloxy-1-propanol. Based on the experimental results, O-bound and C-bound Ru enolate
Asymmetric synthesis of (R)-and (S)-4-methyloctanoic acids. A new route to chiral fatty acids with remote stereocenters.
Mu?oz L, et al.
Tetrahedron Asymmetry, 20(4), 420-424 (2009)
Douglas M Mans et al.
Organic letters, 6(19), 3305-3308 (2004-09-10)
[reaction: see text] The total synthesis of (+)-cocaine is described. An extension of the recently reported proline catalyzed intramolecular enol-exo-aldol reaction to a meso-dialdehyde provided the tropane ring skeleton directly with good enantiomeric excess. The meso-dialdehyde was prepared using a
Synthesis of galactosyl phosphate diester derivatives of nucleosides.
T W Ma et al.
Carbohydrate research, 257(2), 323-330 (1994-05-05)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service