361747
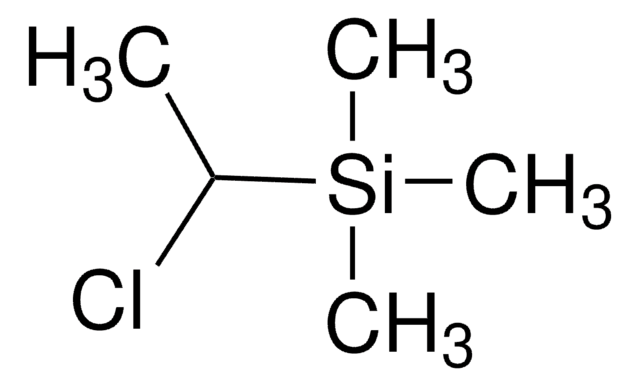
Chlorobis(trimethylsilyl)methane
97%
Synonym(s):
Bis(trimethylsilyl)chloromethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3Si]2CHCl
CAS Number:
Molecular Weight:
194.85
Beilstein:
1736681
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.449 (lit.)
bp
57-60 °C/15 mmHg (lit.)
density
0.892 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
C[Si](C)(C)C(Cl)[Si](C)(C)C
InChI
1S/C7H19ClSi2/c1-9(2,3)7(8)10(4,5)6/h7H,1-6H3
InChI key
XNJGZHVYPBNLEB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Chlorobis(trimethylsilyl)methane can be used as a reagent for the preparation of:
- Para-bis(trimethylsilyl)ethylstyrene (PBTES) monomer, which is used to synthesize corresponding network polymer of styrene.
- Bis(trimethylsilyl)methyl magnesium chloride (Grignard reagent), which is used in the synthesis of bis(trimethylsilyl) allyl compounds by reacting with alkenyl bromide via the Kumada coupling reaction.
- N-[Bis(trimethylsilyl)methyl]heterocumulenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of some silyl mono-and polystyrenes with new properties
Assadi, MG and Hosseinzadeh, F
Designed Monomers and Polymers, 13(2), 181-191 (2010)
Synthesis and Reactivity of N-[Bis (trimethylsilyl) methyl] heterocumulenes
Barbaro G, et al.
The Journal of Organic Chemistry, 60(19), 6032-6039 (1995)
David R Williams et al.
Organic letters, 8(20), 4393-4396 (2006-09-22)
Allylation reagents, which possess geminal bis-trimethylsilyl substitution, are readily prepared from E- or Z-alkenyl bromides. The reactivity of 3,3-bis(trimethylsilyl)-2-methyl-1-propene (1) is described and predominantly provides ene reactions with aldehydes to give alcohol 2 in the presence of BF3.OEt2. Alternatively, Sakurai
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service