361461
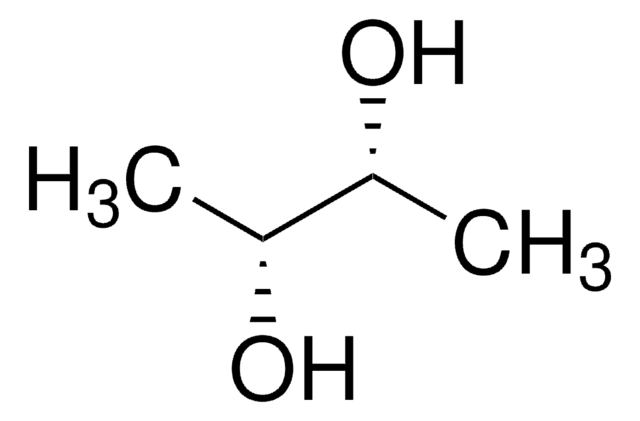
meso-2,3-Butanediol
99%
Synonym(s):
(2R,3S)-2,3-Butanediol, (R,S)-2,3-Butanediol, (erythro-) 2,3-Butanediol, rel-(2R,3S)-2,3-Butanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(OH)CH(OH)CH3
CAS Number:
Molecular Weight:
90.12
Beilstein:
1718900
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
183-184 °C (lit.)
mp
32-34 °C (lit.)
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
C[C@@H](O)[C@H](C)O
InChI
1S/C4H10O2/c1-3(5)4(2)6/h3-6H,1-2H3/t3-,4+
InChI key
OWBTYPJTUOEWEK-ZXZARUISSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
meso-2,3-Butanediol is a diol. 2,3-Butanediol (2,3-DB) exists in three stereoisomeric forms: dextro, levo and meso. 2,3-DB is a crucial chemical feedstock and has wide applications in industry. Production of meso-2,3-butanediol under low oxygen condition by metabolically engineered Escherichia coli is reported. 2,3-Butanediol can be converted to 1,3-butadiene, which is used in the preparation of synthetic rubber. meso-2,3-Butanediol is the source of production of 2-butanol by isolates of lactic acid bacteria.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Payam Ghiaci et al.
FEMS microbiology letters, 360(1), 70-75 (2014-09-02)
2-Butanol has been an issue of industries in many areas, for example, biofuel production (as an advanced alternate fuel), fermented beverages, and food (as taste-altering component). Thus, its source of production, the biological pathway, and the enzymes involved are of
Zheng-Jun Li et al.
Applied microbiology and biotechnology, 87(6), 2001-2009 (2010-05-26)
A metabolically engineered Escherichia coli has been constructed for the production of meso-2,3-butanediol (2,3-BD) under low oxygen condition. Genes responsible for 2,3-BD formation from pyruvate were assembled together to generate a high-copy plasmid pEnBD, in which each gene was transcribed
Yuanzhi He et al.
Molecules (Basel, Switzerland), 23(3) (2018-03-23)
(3S)-Acetoin and (2S,3S)-2,3-butanediol are important platform chemicals widely applied in the asymmetric synthesis of valuable chiral chemicals. However, their production by fermentative methods is difficult to perform. This study aimed to develop a whole-cell biocatalysis strategy for the production of
Borim Kim et al.
Journal of microbiology and biotechnology, 22(9), 1258-1263 (2012-07-21)
2,3-Butanediol (2,3-BD) is a major metabolite produced by Klebsiella pneumoniae KCTC2242, which is a important chemical with wide applications. Three genes important for 2,3-BD biosynthesis acetolactate decarboxylase (budA), acetolactate synthase (budB), and alcohol dehydrogenase (budC) were identified in K. pneumoniae
Hsin-Chih Lai et al.
Journal of leukocyte biology, 92(4), 807-814 (2012-07-18)
The natural compound 2,3-BTD has diverse physiological effects in a range of organisms, including acting as a detoxifying product of liver alcohol metabolism in humans and ameliorating endotoxin-induced acute lung injury in rats. In this study, we reveal that 2,3-BTD
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service