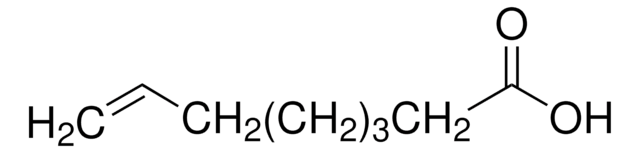358940
6-Heptenoic acid
99%
Synonym(s):
6-Heptanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CH(CH2)4COOH
CAS Number:
Molecular Weight:
128.17
Beilstein:
1747265
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.439 (lit.)
bp
222-224 °C (lit.)
mp
−6.5 °C (lit.)
density
0.946 g/mL at 25 °C (lit.)
functional group
allyl
carboxylic acid
storage temp.
2-8°C
SMILES string
OC(=O)CCCCC=C
InChI
1S/C7H12O2/c1-2-3-4-5-6-7(8)9/h2H,1,3-6H2,(H,8,9)
InChI key
RWNJOXUVHRXHSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
6-Heptenoic acid is a straight-chain terminal alkenoic acid. Electrochemical oxidation of 6-heptenoic acid adsorbed on Pt(111) electrode surface has been reported. Preparation of 6-heptenoic acid has been reported.
Application
6-Heptenoic acid may be used in the preparation of 6-(iodomethyl)-hexanolide, via iodo-lactonization.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
1, 2, 6-Tribromohexane, 5-Hexenylmagnesium Bromide, and 6-Heptenoic Acid1.
Lamb RC and Ayers PW.
The Journal of Organic Chemistry, 27(4), 1441-1442 (1962)
Preparation of seven-membered and medium-ring lactones by iodo lactonization.
Simonot B and Rousseau G.
The Journal of Organic Chemistry, 58(1), 4-5 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service