All Photos(2)
About This Item
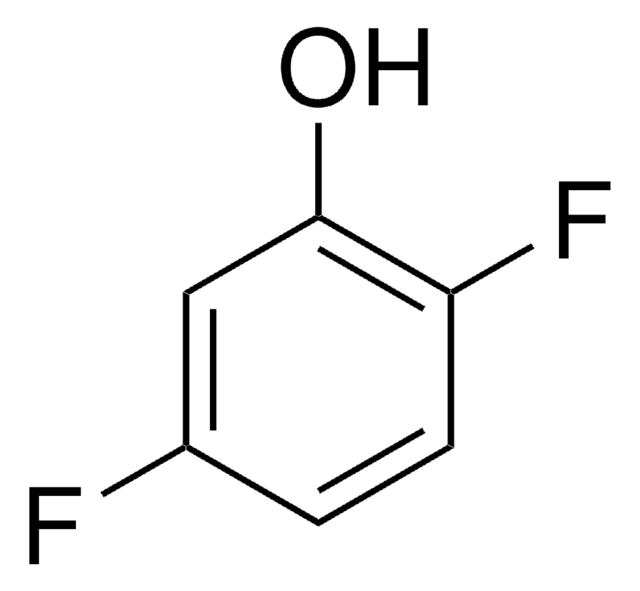
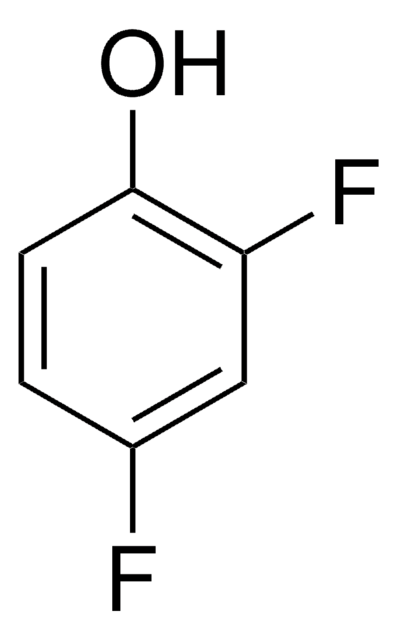
Linear Formula:
F3C6H2OH
CAS Number:
Molecular Weight:
148.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
33-37 °C (lit.)
functional group
fluoro
SMILES string
Oc1c(F)ccc(F)c1F
InChI
1S/C6H3F3O/c7-3-1-2-4(8)6(10)5(3)9/h1-2,10H
InChI key
QSFGUSFDWCVXNR-UHFFFAOYSA-N
General description
2,3,6-Trifluorophenol is a fluorinated phenol. Biotransformation of 2,3,6-trifluorophenol has been studied by 19FNMR technique.
Application
2,3,6-Trifluorophenol may be employed as model compound to investigate the site-selective functionalization of halogen-bearing phenols by organometallic approach.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M G Boersma et al.
Journal of industrial microbiology & biotechnology, 26(1-2), 22-34 (2001-09-11)
Of all NMR-observable isotopes 19F is the one most convenient for studies on the biodegradation of environmental pollutants and especially for fast initial metabolic screening of newly isolated organisms. In the past decade we have identified the 19F NMR characteristics
The site-selective functionalization of halogen-bearing phenols: an exercise in diversity-oriented organometallic synthesis.
Marzi E and Schlosser M.
Tetrahedron, 61(13), 3393-3401 (2005)
Matthew G Romei et al.
Science (New York, N.Y.), 367(6473), 76-79 (2020-01-04)
Rotation around a specific bond after photoexcitation is central to vision and emerging opportunities in optogenetics, super-resolution microscopy, and photoactive molecular devices. Competing roles for steric and electrostatic effects that govern bond-specific photoisomerization have been widely discussed, the latter originating
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service