All Photos(1)
About This Item
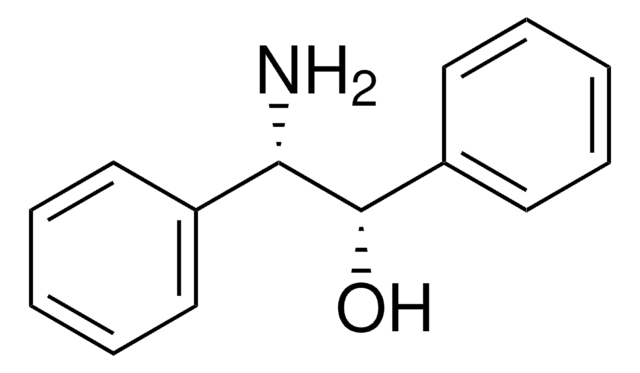
Linear Formula:
C6H5CH(NH2)CH(C6H5)OH
CAS Number:
Molecular Weight:
213.28
Beilstein:
1212828
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
optical activity
[α]25/D +7.0°, c = 0.6 in ethanol
mp
142-144 °C (lit.)
functional group
amine
hydroxyl
phenyl
SMILES string
N[C@@H]([C@@H](O)c1ccccc1)c2ccccc2
InChI
1S/C14H15NO/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-14,16H,15H2/t13-,14+/m1/s1
InChI key
GEJJWYZZKKKSEV-KGLIPLIRSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(1S,2R)-(+)-2-Amino-1,2-diphenylethanol can be used:
- To prepare vanadium(V) Schiff base complexes, which are used as catalysts in the oxidation of sulfides and olefins.
- To prepare chiral selectors, which are immobilized on aminated silica gel, applicable as chiral stationary phase in HPLC.
- To immobilize on the frame of α-zirconium phosphate to yield layered zirconium phosphonates, which are used in the heterogeneous catalysis.
- As a chiral auxiliary in the preparation of homopropargylic alcohols from aliphatic and aromatic aldehydes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vanadium (V) complexes with chiral tridentate Schiff base ligands derived from 1S, 2R (+)-2-amino-1, 2-diphenylethanol and with acetohydroxamate co-ligand: Synthesis, characterization and catalytic activity in the oxidation of prochiral sulfides and olefins
Romanowski G, et al.
J. Mol. Catal. A: Chem., 381, 148-160 (2014)
Zirconium phosphonate immobilized chiral amino alcohol for heterogeneous enantioselective addition of diethylzinc to benzaldehyde
Zheng B, et al.
Catalysis Communications, 8(12), 1923-1928 (2007)
Asymmetric indium-mediated synthesis of homopropargylic alcohols
Hirayama LC, et al.
Tetrahedron Letters, 47(29), 5173-5176 (2006)
Chuan-Qi Yin et al.
Chirality, 21(4), 442-448 (2008-07-26)
A chiral selector was prepared through the reaction between (1S,2R)-(+)-2-amino-1,2-diphenylethanol and phenyl isocyanate. This selector was immobilized on aminated silica gel, respectively, with bifunctional group linkers of 1,4-phenylene diisocyanate, methylene-di-p-phenyl diisocyanate, and terephthaloyl chloride to produce corresponding three chiral stationary
Michael L Berger et al.
Bioorganic & medicinal chemistry, 17(9), 3456-3462 (2009-04-07)
We resolved 1,2-diphenylethylamine (DPEA) into its (S)- and (R)-enantiomer and used them as precursors for synthesis of (S)- and (R)-1-(1,2-diphenylethyl)piperidine, flexible homeomorphs of the NMDA channel blocker MK-801. We also describe the synthesis of the dicyclohexyl analogues of DPEA. These
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service