All Photos(1)
About This Item
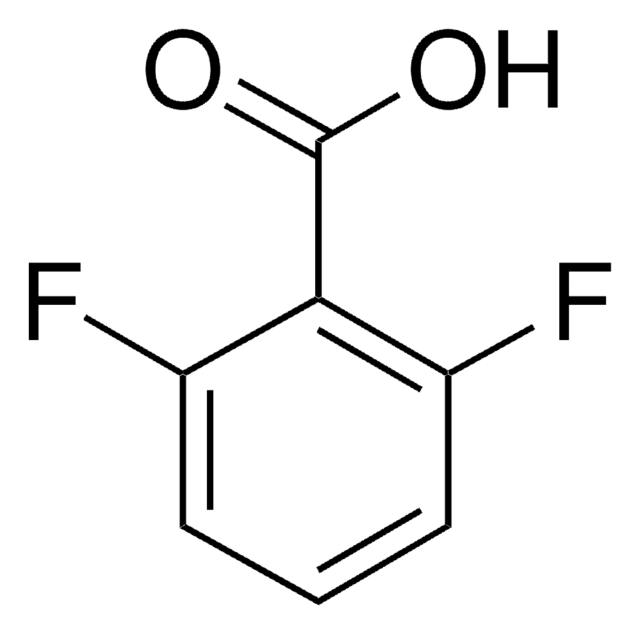
Linear Formula:
F3C6H2CO2H
CAS Number:
Molecular Weight:
176.09
Beilstein:
3257609
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
94-96 °C (lit.)
functional group
carboxylic acid
fluoro
SMILES string
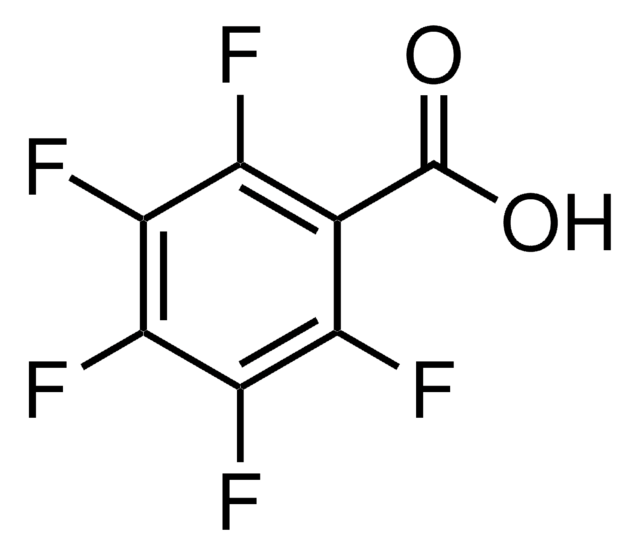
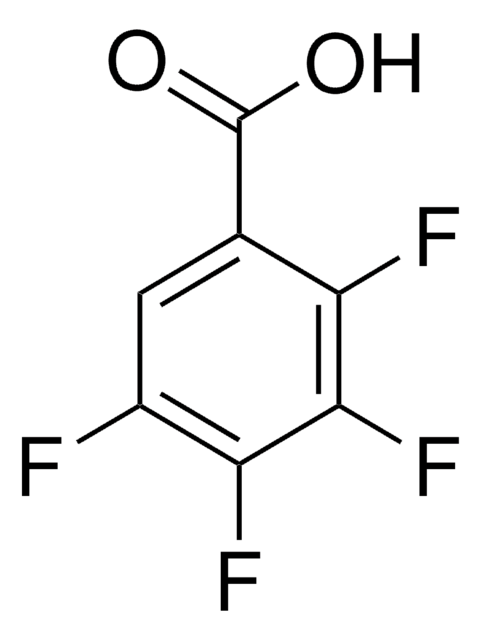
OC(=O)c1cc(F)c(F)cc1F
InChI
1S/C7H3F3O2/c8-4-2-6(10)5(9)1-3(4)7(11)12/h1-2H,(H,11,12)
InChI key
AKAMNXFLKYKFOJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4,5-Trifluorobenzoic acid is an important quinolone antibacterial intermediate.
Application
2,4,5-Trifluorobenzoic acid was used in preparation of 5-fluoro-2-methoxy-4-methylaminbenzamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An Expedient Route to the Quinolone Antibacterial Intermediate, 2, 4, 5-Trifluorobenzoic Acid.
O'Reilly NJ, et al.
Synlett, 1990(10), 609-610 (1990)
Yoshimi Hirokawa et al.
Chemical & pharmaceutical bulletin, 50(7), 941-959 (2002-07-20)
In search of a dopamine D2 and serotonin 5-HT3 receptors dual antagonist as a potential broad antiemetic agent, a number of benzamides were prepared from 4-amino-5-chloro-2-methoxybenzoic acid derivatives and 6-amino-1,4-dialkylhexahydro-1,4-diazepines and evaluated for their binding affinity for the dopamine D2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service