302481
4-Pentyn-1-ol
97%
Synonym(s):
(3-Hydroxypropyl)acetylene, 1-Hydroxy-4-pentyne, 1-Pentyn-5-ol, 5-Hydroxy-1-pentyne, Pent-4-yn-1-ol, Pent-4-yne-1-ol, Pentyne alcohol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
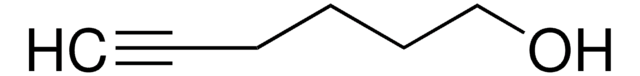
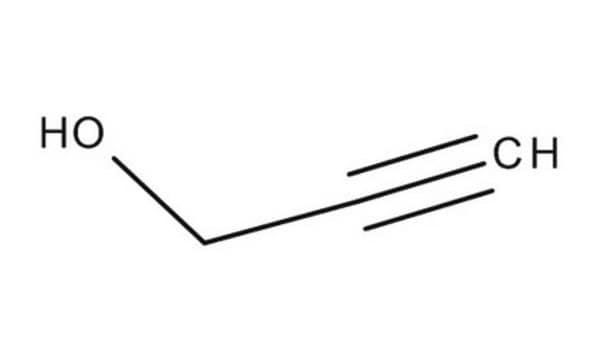
HC≡CCH2CH2CH2OH
CAS Number:
Molecular Weight:
84.12
Beilstein:
1736712
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.445 (lit.)
bp
154-155 °C (lit.)
density
0.904 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OCCCC#C
InChI
1S/C5H8O/c1-2-3-4-5-6/h1,6H,3-5H2
InChI key
CRWVOXFUXPYTRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Mechanism of (THF)W(CO)5-promoted endo- and exo-cycloisomerization of 4-pentyn-1-ol was studied.
Application
4-Pentyn-1-ol was used in preparation of 3-pent-4-ynyloxy phthalonitrile. It was also used as starting reagent in stereoselective total synthesis of antimicrobial marine metabolites, ieodomycin A and B.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tomás Sordo et al.
Journal of the American Chemical Society, 127(3), 944-952 (2005-01-20)
New solvent-assisted mechanistic routes were located for the (THF)W(CO)5-promoted endo- and exo-cycloisomerization of 4-pentyn-1-ol using the B3LYP/6-31G (with the LANL2DZ relativistic pseudopotential for W) theory level. A mixed model was used by explicitly including a THF molecule as a component
Sayantan Das et al.
The Journal of organic chemistry, 78(14), 7274-7280 (2013-06-21)
Stereoselective total synthesis of antimicrobial marine metabolites ieodomycin A and B have been accomplished starting from commercially available 4-pentyn-1-ol featuring strategic application of the Negishi reaction, Kumada coupling, and Crimmins acetate aldol. This revises the proton NMR spectra of ieodomycin
Yuan Wang et al.
Proceedings of the National Academy of Sciences of the United States of America, 116(24), 12094-12102 (2019-05-31)
As the most common RNA cap in eukaryotes, the 7-methylguanosine (m7G) cap impacts nearly all processes that a messenger RNA undergoes, such as splicing, polyadenylation, nuclear export, translation, and degradation. The metabolite and redox agent, nicotinamide adenine diphosphate (NAD+), can
Zeliha Kanat et al.
Dalton transactions (Cambridge, England : 2003), 43(23), 8654-8663 (2014-04-26)
In order to obtain nonperipherally tetra terminal alkynyl substituted phthalocyanines (Pcs), new 3-pent-4-ynyloxy phthalonitrile (3) was prepared by the nucleophilic displacement reaction of 3-nitrophthalonitrile (1) and 4-pentyn-1-ol (2) and then cyclotetramerization was attained in the presence of zinc acetate, cobalt
André S de Oliveira et al.
PloS one, 14(9), e0223017-e0223017 (2019-09-27)
The West Nile Virus (WNV) NS2B-NS3 protease is an attractive target for the development of therapeutics against this arboviral pathogen. In the present investigation, the screening of a small library of fifty-eight synthetic compounds against the NS2-NB3 protease of WNV
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service