296066
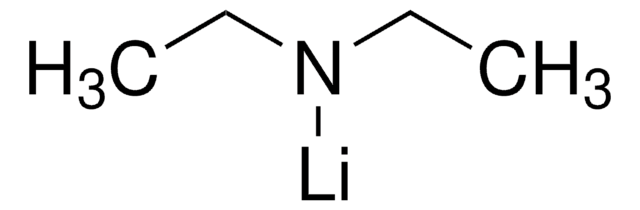
Lithium dimethylamide
95%
Synonym(s):
Lithiodimethylamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2NLi
CAS Number:
Molecular Weight:
51.02
Beilstein:
3618233
EC Number:
MDL number:
UNSPSC Code:
12352111
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
functional group
amine
SMILES string
[Li]N(C)C
InChI
1S/C2H6N.Li/c1-3-2;/h1-2H3;/q-1;+1
InChI key
YDGSUPBDGKOGQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Lithium dimethylamide is used as a reagent in the synthesis of boron-nitrogen heterocycles and 2,2′?diborabiphenyl. It is a deprotecting agent which is employed in demethylation of bis(imino)pyridine ferrous dichloride for synthesizing ferrous amide-ate complexes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Pyr. Sol. 1 - Skin Corr. 1B - Water-react 2
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bis (imino) pyridine ligand deprotonation promoted by a transient iron amide.
Bouwkamp MW, et al.
Inorganic Chemistry, 45(1), 2-4 (2006)
2, 2′-Diborabiphenyl: A Lewis Acid Analogue of 2, 2′-Bipyridine.
Emslie DJH, et al.
Angewandte Chemie (International Edition in English), 115(11), 1290-1293 (2003)
Triphenylene analogues with B2N2C2 cores: synthesis, structure, redox behavior, and photophysical properties.
Jaska CA, et al.
Journal of the American Chemical Society, 128(33), 10885-10896 (2006)
Richard O'Donoghue et al.
Dalton transactions (Cambridge, England : 2003), 46(47), 16551-16561 (2017-11-22)
Herein we describe an efficient low temperature (60-160 °C) plasma enhanced atomic layer deposition (PEALD) process for gallium oxide (Ga
Lukas Mai et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(31), 7489-7500 (2019-03-15)
New precursor chemistries for the atomic layer deposition (ALD) of aluminium oxide are reported as potential alternatives to the pyrophoric trimethylaluminium (TMA) which is to date a widely used Al precursor. Combining the high reactivity of aluminium alkyls employing the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service