290785
Luperox® LP, Lauroyl peroxide
≥98%
Synonym(s):
Lauroyl peroxide, Di(dodecanoyl) peroxide, DiDodecanoyl Peroxide, Dilauroyl peroxide, Dodecanoyl peroxide
About This Item
Recommended Products
vapor density
13.7 (vs air)
Assay
≥98%
form
solid
reaction suitability
reagent type: oxidant
mp
53-57 °C (lit.)
storage temp.
2-8°C
SMILES string
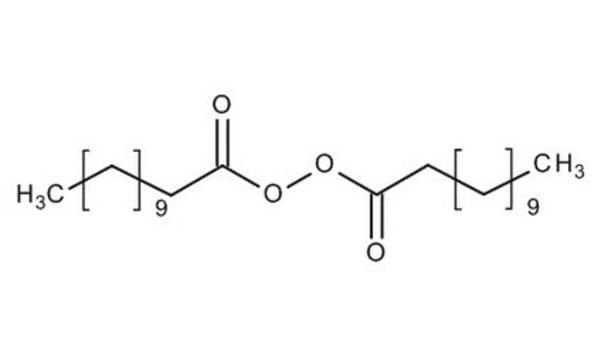
CCCCCCCCCCCC(=O)OOC(=O)CCCCCCCCCCC
InChI
1S/C24H46O4/c1-3-5-7-9-11-13-15-17-19-21-23(25)27-28-24(26)22-20-18-16-14-12-10-8-6-4-2/h3-22H2,1-2H3
InChI key
YIVJZNGAASQVEM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- A long chain branching(LCB) introducer in polypropylene to improve its mechanical recycling process.
- A mild oxidant and efficient hydrogen abstractor in the preparation of disulfide compounds through dimerization of corresponding dithiocarbamates.
- An initiator in the preparation of ion-imprinted polymers(IIPs).
- An initiator and oxidant to construct erythrina ring system via oxidative radical cyclizations of enamide in the presence of nBu3SnH.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Org. Perox. D
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service