276286
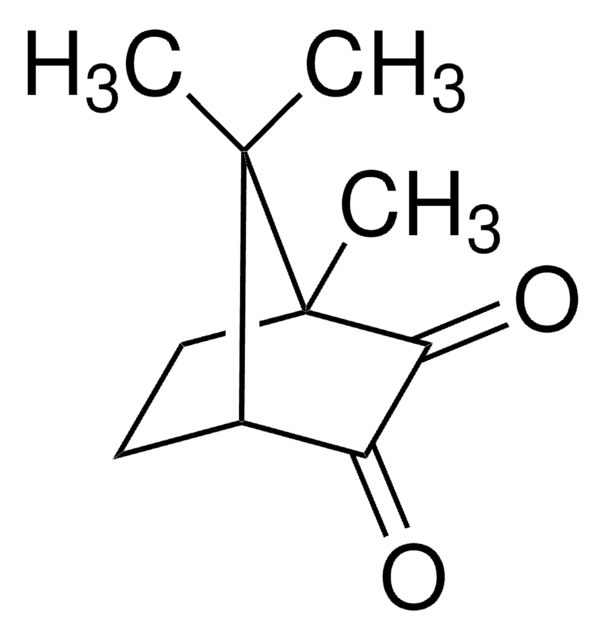
(1R)-(−)-Camphorquinone
99%
Synonym(s):
(1R)-(−)-2,3-Bornanedione, 2,3-Bornanedione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H14O2
CAS Number:
Molecular Weight:
166.22
Beilstein:
2327696
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]20/D −101°, c = 2 in toluene
mp
200-203 °C (lit.)
functional group
ketone
SMILES string
CC1(C)[C@@H]2CC[C@@]1(C)C(=O)C2=O
InChI
1S/C10H14O2/c1-9(2)6-4-5-10(9,3)8(12)7(6)11/h6H,4-5H2,1-3H3/t6-,10+/m1/s1
InChI key
VNQXSTWCDUXYEZ-LDWIPMOCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(1R)-(−)-Camphorquinone can be used as a chiral starting material for the preparation of:
- α-Hydroxycamphors by selective reduction of keto groups using various vegetables.
- Camphor-1,2-diamine platinum(II) complexes for DNA interaction studies.
- Camphoric anhydride by unsensitized photo-oxidation in the presence of oxygen and polar solvents.
- Camphorquinone-based chiral homoallylic amine, which is reacted with aldehydes to produce homoallylic primary amines via imine formation followed by 2-azonia-Cope rearrangement.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of camphoric anhydride via unsensitized photo-oxidation of camphorquinone
Shun-Jun J, et al.
Synthetic Communications, 32(11), 1659-1663 (2002)
Enantioselective transfer aminoallylation: synthesis of optically active homoallylic primary amines.
Masaharu Sugiura et al.
Journal of the American Chemical Society, 128(34), 11038-11039 (2006-08-24)
A camphorquinone-derived chiral homoallylic amine was found to react with various aldehydes via imine formation and asymmetric 2-azonia-Cope rearrangement to give optically active homoallylic primary amines. A practical level of enantioselectivity with high functional group tolerance has been attained in
Stereoselective reduction of ketones by various vegetables
Utsukihara T, et al.
Journal of Molecular Catalysis. B, Enzymatic, 41(3-4), 103-109 (2006)
Angel M Montaña et al.
Bioorganic & medicinal chemistry, 16(4), 1721-1737 (2007-11-27)
The platinum(II) complex cis-[(1S,2R,3S)-1,7,7-trimethylbicyclo[2.2.1]heptane-2,3-diamine]dichloroplatinum(II) (1) and its enantiomer (2) have been synthesized and physically and spectroscopically characterized. To obtain the enantiopure complexes the chiral pool approach was applied. The synthetic pathway has four steps, starting from (+/-)-diphenylethylenediamine (DPEDA) (3) and
Tetrahedron Asymmetry, 17, 1179-1179 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service