256005
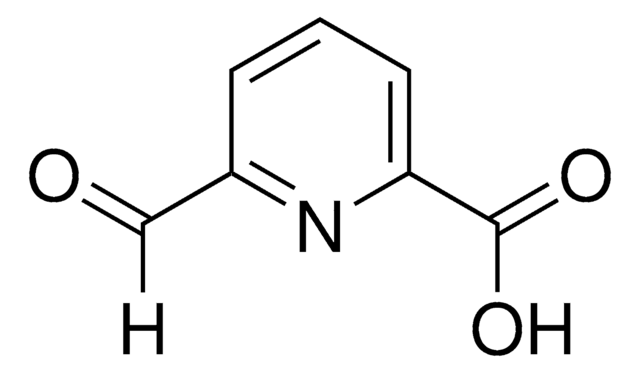
2,6-Pyridinedicarboxaldehyde
97%
Synonym(s):
2,6-Bis(formyl)pyridine, 2,6-Diformylpyridine, 2,6-Pyridinedialdehyde, Pyridine-2,6-dicarbaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H5NO2
CAS Number:
Molecular Weight:
135.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
152-154 °C/103 mmHg (lit.)
mp
124-125 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
[H]C(=O)c1cccc(n1)C([H])=O
InChI
1S/C7H5NO2/c9-4-6-2-1-3-7(5-10)8-6/h1-5H
InChI key
PMWXGSWIOOVHEQ-UHFFFAOYSA-N
General description
2,6-Pyridinedicarboxaldehyde can serve as a building block in the synthesis of various organic compounds.
Application
2,6-Pyridinedicarboxaldehyde has been used in preparation of:
- functionalized resin Amberlite XAD-4
- boron-dipyrromethene (BODIPY)-based fluorescence probe with a N,N′-(pyridine-2, 6-diylbis(methylene))-dianiline substituent
- novel N-heterocyclic chitosan aerogel derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cennet Karadaş et al.
Food chemistry, 141(2), 655-661 (2013-06-26)
The synthesis and characterization of the resin Amberlite XAD-4 functionalized with 2,6-pyridinedicarboxaldehyde and its application in an on-line system for the preconcentration of cadmium, cobalt, copper, lead and manganese prior to determination using flame atomic absorption spectrometry (FAAS) is proposed.
Hua Lu et al.
The journal of physical chemistry. A, 113(51), 14081-14086 (2009-12-03)
A boron-dipyrromethene (BODIPY)-based fluorescence probe with a N,N'-(pyridine-2, 6-diylbis(methylene))-dianiline substituent (1) has been prepared by condensation of 2,6-pyridinedicarboxaldehyde with 8-(4-amino)-4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene and reduction by NaBH(4). The sensing properties of compound 1 toward various metal ions are investigated via fluorometric titration in
Santosh Kumar et al.
International journal of biological macromolecules, 45(4), 330-337 (2009-08-12)
The novel N-heterocyclic chitosan aerogel derivatives were prepared by reacting 79% deacetylated chitosan separately with 4-pyridinecarboxaldehyde and 2,6-pyridinedicarboxaldehyde followed by subsequent solvent exchange into acetone, filteration and lyophilization. The identity of the Schiff bases was confirmed by UV-vis absorption spectroscopy
Muhammad Saleem et al.
Journal of fluorescence, 26(1), 11-22 (2015-11-21)
Herein, we reported the ditriazole Schiff base derivative 1 and evaluated its photophysical properties on induction of varieties of metal ions including Na(+), Ag(+), Ni(2+), Mn(2+), Pd(2+), Co(2+), Hg(2+), Cu(2+), Pb(2+), Cd(2+), Zn(2+), Sn(2+), Fe(2+), Fe(3+), Cr(3+) and Al(3+), in
Muhammad Hanif et al.
Molecules (Basel, Switzerland), 24(2) (2019-01-19)
The present study focuses on the design and synthesis of a cage-like organic skeleton containing two triazole rings jointed via imine linkage. These molecules can act as urease inhibitors. The in-vitro urease inhibition screening results showed that the combination of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service