254037
Chloro(triphenylphosphine)gold(I)
≥99.9% trace metals basis
Synonym(s):
(Ph3P)AuCl, Triphenylphosphinegold(I) chloride
About This Item
Recommended Products
Quality Level
Assay
≥99.9% trace metals basis
form
solid
reaction suitability
core: gold
reagent type: catalyst
SMILES string
Cl[Au].c1ccc(cc1)P(c2ccccc2)c3ccccc3
InChI
1S/C18H15P.Au.ClH/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;/h1-15H;;1H/q;+1;/p-1
InChI key
IFPWCRBNZXUWGC-UHFFFAOYSA-M
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Chloro[tris(para-trifluoromethylphenyl)phosphine]gold(I) 99%](/deepweb/assets/sigmaaldrich/product/structures/250/453/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e/640/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e.png)

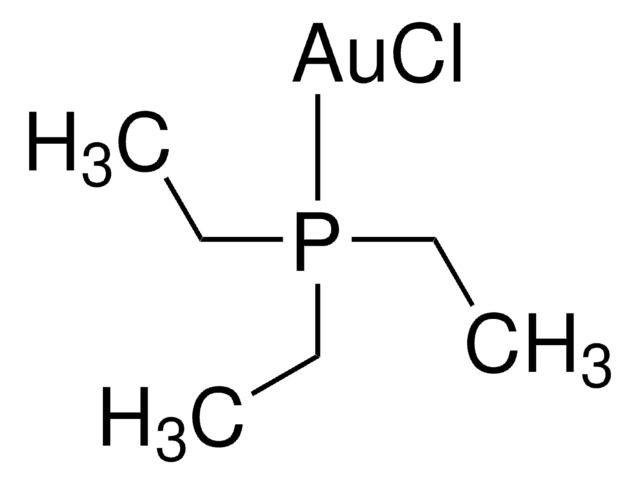


![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
gold(I) (2:1) toluene adduct](/deepweb/assets/sigmaaldrich/product/structures/104/897/81ee3e56-c988-4d0f-9614-1269b470316d/640/81ee3e56-c988-4d0f-9614-1269b470316d.png)

![Chloro[2-dicyclohexyl(2′,4′,6′-trisopropylbiphenyl)phosphine]gold(I)](/deepweb/assets/sigmaaldrich/product/structures/253/590/7ea3a0c9-1b4c-4e68-8fa0-5e764f73519a/640/7ea3a0c9-1b4c-4e68-8fa0-5e764f73519a.png)
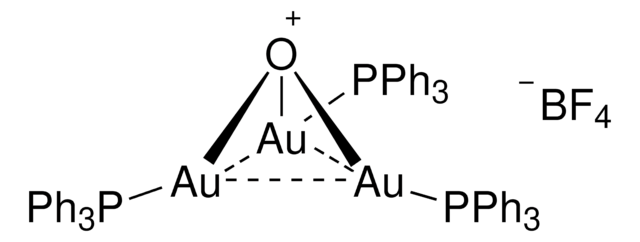
![Chloro[tris(2,4-di-tert-butylphenyl)phosphite]gold](/deepweb/assets/sigmaaldrich/product/structures/386/294/6df0db46-002b-4599-ad6c-451c419a3fc5/640/6df0db46-002b-4599-ad6c-451c419a3fc5.png)
![(Acetonitrile)[(2-biphenyl)di-tert-butylphosphine]gold(I) hexafluoroantimonate](/deepweb/assets/sigmaaldrich/product/structures/216/222/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9/640/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9.png)