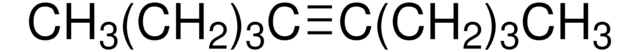All Photos(1)
About This Item
Linear Formula:
CH3CH2CH2C≡CCH2CH2CH3
CAS Number:
Molecular Weight:
110.20
Beilstein:
1732138
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39010412
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
35 mmHg ( 37.7 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.425 (lit.)
bp
131-132 °C (lit.)
mp
−103 °C (lit.)
density
0.751 g/mL at 25 °C (lit.)
SMILES string
CCCC#CCCC
InChI
1S/C8H14/c1-3-5-7-8-6-4-2/h3-6H2,1-2H3
InChI key
GZTNBKQTTZSQNS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Octyne, also known as dipropylethyne can be used as a versatile building block in organic synthesis. The kinetics of the stereoselective semi-hydrogenation of 4-octyne (in tetrahydrofuran) was studied.
Application
4-Octyne, an electron-rich dialkylacetylene, was used in the synthesis of highly substituted 1,3-dienes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
84.2 °F - closed cup
Flash Point(C)
29 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cyclopentadienone synthesis by rhodium (I)-catalyzed [3+ 2] cycloaddition reactions of cyclopropenones and alkynes
PA Wender, et al.
Journal of the American Chemical Society, 128, 14814-14815 (2006)
Alexander M Kluwer et al.
Journal of the American Chemical Society, 127(44), 15470-15480 (2005-11-03)
The kinetics of the stereoselective semi-hydrogenation of 4-octyne in THF by the highly active catalyst [Pd{(m,m'-(CF(3))(2)C(6)H(3))-bian}(ma)] (2) (bian = bis(imino)acenaphthene; ma = maleic anhydride) has been investigated. The rate law under hydrogen-rich conditions is described by r = k[4-octyne](0.65)[Pd][H(2)], showing
Chengxiang Zhou et al.
The Journal of organic chemistry, 71(8), 3184-3191 (2006-04-08)
The Pd(II)-catalyzed reaction of arylboronic acids and internal alkynes provides a convenient route to a wide variety of tetrasubstituted olefins. The reaction is conducted in DMSO using molecular O2 as an oxidant in the absence of any base. The reaction
Davide Albani et al.
Nature communications, 9(1), 2634-2634 (2018-07-08)
Ensemble control has been intensively pursued for decades to identify sustainable alternatives to the Lindlar catalyst (PdPb/CaCO3) applied for the partial hydrogenation of alkynes in industrial organic synthesis. Although the geometric and electronic requirements are known, a literature survey illustrates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service