242403
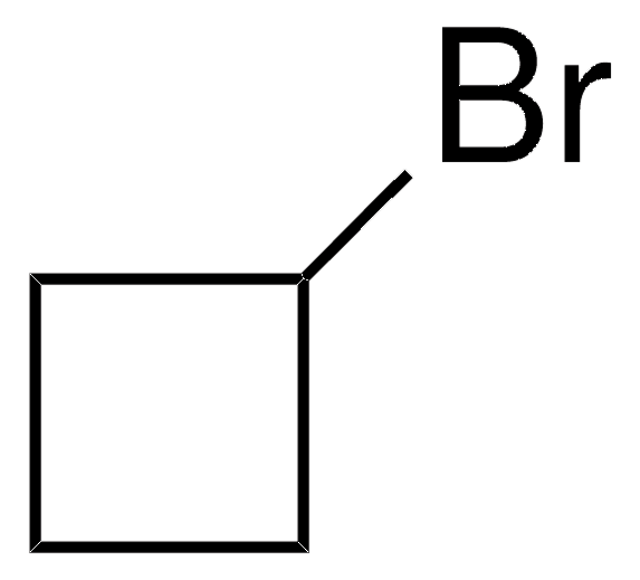
(Bromomethyl)cyclopropane
97%
Synonym(s):
Cyclopropylmethyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C3H5CH2Br
CAS Number:
Molecular Weight:
135.00
Beilstein:
605296
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.457 (lit.)
bp
105-107 °C (lit.)
density
1.392 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
BrCC1CC1
InChI
1S/C4H7Br/c5-3-4-1-2-4/h4H,1-3H2
InChI key
AEILLAXRDHDKDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The coupling reaction of (bromomethyl)cyclopropane with phenylmagnesium bromide was studied.
Application
(Bromomethyl)cyclopropane was used as a synthetic building block for the introduction of the cyclopropylmethyl group. It was also used in the synthesis of 1,4-dienes via iron-catalyzed cross-coupling with alkenyl Grignard reagents.
accessory
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
105.8 °F
Flash Point(C)
41 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Robin B Bedford et al.
Chemical communications (Cambridge, England), (33)(33), 4161-4163 (2005-08-16)
Mixtures of iron(III) chloride and appropriate amine ligands are active catalysts for the coupling of aryl Grignard reagents with primary and secondary alkyl halide substrates bearing beta-hydrogens, under mild and simple reaction conditions.
Subramaniam Ananthan et al.
Journal of medicinal chemistry, 47(6), 1400-1412 (2004-03-05)
A series of pyridomorphinans derived from naloxone, oxymorphone, and hydromorphone (7a-k) were synthesized and evaluated for binding affinity at the opioid delta, micro, and kappa receptors in brain membranes using radioligand binding assays and for functional activity in vitro using
Yong-Jin Wu et al.
Bioorganic & medicinal chemistry letters, 14(8), 1991-1995 (2004-03-31)
(S)-N-[1-(4-Cyclopropylmethyl-3,4-dihydro-2H-benzo[1,4]oxazin-6-yl)-ethyl]-3-(2-fluoro-phenyl)-acrylamide ((S)-2) was identified as a potent and efficacious KCNQ2 opener. This compound demonstrated significant activity in reducing neuronal hyperexcitability in rat hippocampal slices, and the inhibition mediated by (S)-2 was reversed by the KCNQ blocker linopirdine.
Iron-catalyzed cross-coupling of alkyl halides with alkenyl grignard reagents.
Amandine Guérinot et al.
Angewandte Chemie (International ed. in English), 46(34), 6521-6524 (2007-07-28)
Ning Xi et al.
Bioorganic & medicinal chemistry letters, 14(2), 377-381 (2003-12-31)
A novel series of piperazines appended to a succinamide backbone were synthesized and found to have a high affinity for the melanocortin-4 receptor (IC(50)s ranging from <0.1 to 200 nM). Both agonists and antagonists of MC4R were prepared by modifying
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service