All Photos(1)
About This Item
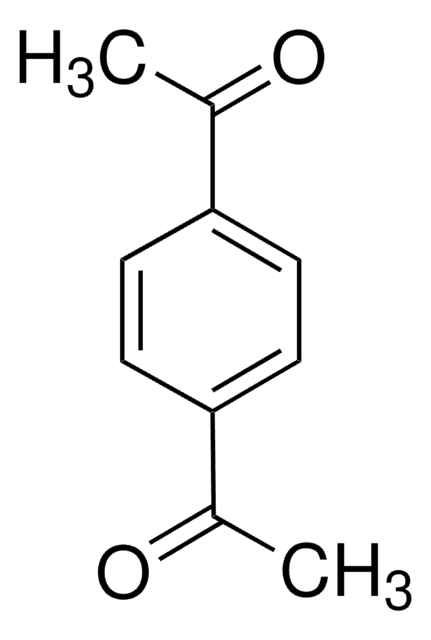
Linear Formula:
C6H4(COCH3)2
CAS Number:
Molecular Weight:
162.19
Beilstein:
1862907
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
110 °C/0.1 mmHg (lit.)
mp
39-41 °C (lit.)
solubility
dichloromethane: soluble 50 mg/mL, clear, colorless to yellow
fluorescence
λex 355 nm; λem 455 nm (Amine adducts)
functional group
ketone
SMILES string
CC(=O)c1ccccc1C(C)=O
InChI
1S/C10H10O2/c1-7(11)9-5-3-4-6-10(9)8(2)12/h3-6H,1-2H3
InChI key
LVQFKRXRTXCQCZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,2-Diacetylbenzene, an aromatic hydrocarbon, is a protein-reactive γ-diketone metabolite of the neurotoxic solvent 1,2-diethylbenzene.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Min-Sun Kim et al.
Chemico-biological interactions, 194(2-3), 139-147 (2011-10-25)
1,2-Diacetylbenzene (DAB) is a neurotoxic minor metabolite of 1,2-diethylbenzene or naphthalene reaction product with OH radical. DAB causes central and peripheral neuropathies that lead to motor neuronal deficits. However, the potent effects and molecular mechanisms of DAB on neural progenitor
D D Tshala-Katumbay et al.
Acta neuropathologica, 109(4), 405-410 (2005-03-11)
The aromatic gamma-diketone 1,2-diacetylbenzene (1,2-DAB), the putative active metabolite of the organic solvent 1,2-diethylbenzene, forms blue-colored polymeric protein adducts and induces the formation of amyotrophic lateral sclerosis (ALS)-like giant, intraspinal neurofilamentous axonal swellings in Sprague Dawley rats. The pathogenetic mechanism
Min Kyeong Kim et al.
Journal of toxicology and environmental health. Part A, 70(15-16), 1336-1343 (2007-07-27)
Organic solvents are ubiquitous in industrial and household surroundings, and thus individuals are easily exposed. 1,2-Diethylbenzene (DEB) is one of organic solvents contained in gasoline or jet fuels. DEB is absorbed by dermal or inhalation routes, metabolized by cytochrome P-450
Min-Sun Kim et al.
Toxicology, 243(1-2), 216-223 (2007-12-08)
Environmental substances or metabolites induce neuronal damage through oxidative stress. Environmental organic solvent metabolite, 1,2-diacetylbenzene (1,2-DAB), treated rats develop limb weakness with neuropathological damage in both the central and peripheral nervous systems. In this experiment, we examined the relevance of
Xin-Jun Yu et al.
Molecules (Basel, Switzerland), 25(6) (2020-03-18)
In the present study, a pyridoxal-5'-phosphate (PLP)-dependent L-aspartate-α-decarboxylase from Tribolium castaneum (TcPanD) was selected for protein engineering to efficiently produce β-alanine. A mutant TcPanD-R98H/K305S with a 2.45-fold higher activity than the wide type was selected through error-prone PCR, site-saturation mutagenesis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service