241717
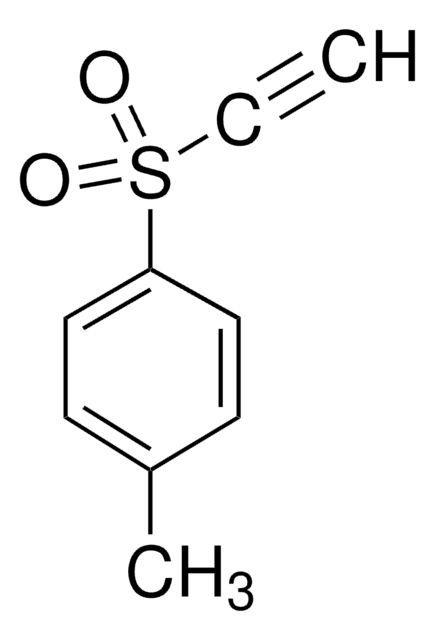
Phenyl vinyl sulfone
99%
Synonym(s):
(Ethenesulfonyl)benzene, (Ethenylsulfonyl)benzene, Ethenyl phenyl sulfone, Vinylsulfonylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SO2CH=CH2
CAS Number:
Molecular Weight:
168.21
Beilstein:
1906894
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
67-69 °C (lit.)
solubility
organic solvents: soluble(lit.)
functional group
sulfone
SMILES string
C=CS(=O)(=O)c1ccccc1
InChI
1S/C8H8O2S/c1-2-11(9,10)8-6-4-3-5-7-8/h2-7H,1H2
InChI key
UJTPZISIAWDGFF-UHFFFAOYSA-N
Gene Information
human ... LOC129293(129293)
Looking for similar products? Visit Product Comparison Guide
General description
Phenyl vinyl sulfone, a synthetic inhibitor of cysteine protease, exhibits antihelminthic and antiprotozoal properties.
Application
Phenyl vinyl sulfone was used as a common reagent in a variety of cycloaddition reactions to form cyclopropanes, cyclohexenes and cyclooctadienes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron, 49, 5495-5495 (1993)
Aldrichimica Acta, 15, 34-34 (1982)
Tetrahedron Letters, 34, 7583-7583 (1993)
Yumiko Nakayama et al.
Environmental sciences : an international journal of environmental physiology and toxicology, 12(6), 371-379 (2006-04-13)
To investigate the safe handling of an industrial product, phenyl vinyl sulfone (PVS), which has an extremely high potential for dermal sensitization at low concentrations and positive mutagenicity, the maximum no-effect concentration for dermal deposits was obtained from dermal sensitization
Catalytic enantioselective reduction of beta,beta-disubstituted vinyl phenyl sulfones by using bisphosphine monoxide ligands.
Jean-Nicolas Desrosiers et al.
Angewandte Chemie (International ed. in English), 46(31), 5955-5957 (2007-06-07)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service