All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H8O
CAS Number:
Molecular Weight:
168.19
Beilstein:
121100
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
154-155 °C/20 mmHg (lit.)
mp
80-82 °C (lit.)
solubility
acetic acid: soluble(lit.)
benzene: soluble(lit.)
diethyl ether: soluble(lit.)
ethanol: soluble(lit.)
water: insoluble(lit.)
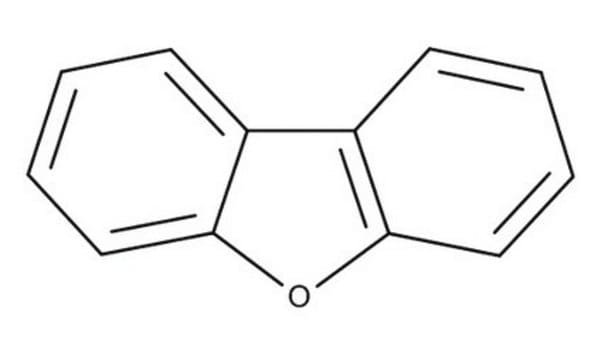
SMILES string
c1ccc2c(c1)oc3ccccc23
InChI
1S/C12H8O/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H
InChI key
TXCDCPKCNAJMEE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dibenzofuran has been converted to a vacuum-sublimable, electron-transporting host material for blue-green electrophosphorescent molecule, iridium (III) bis(4,6-(di-fluorophenyl)pyridinato-N,C2′)picolinate. Biodegradation of dibenzofuran (DF) and its structural analogs by a newly isolated Agrobacterium sp. PH-08 has been reported.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
266.0 °F - closed cup
Flash Point(C)
130 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Paul A Vecchi et al.
Organic letters, 8(19), 4211-4214 (2006-09-08)
Dibenzofuran (DBF) is converted to a vacuum-sublimable, electron-transporting host material via 2,8-substitution with diphenylphosphine oxide moieties. Close pi-pi stacking and the inductive influence of P=O moieties impart favorable electron-transport properties without lowering the triplet energy. A maximum external quantum efficiency
T T Le et al.
Journal of applied microbiology, 116(3), 542-553 (2013-11-28)
To demonstrate the biodegradation of dibenzofuran (DF) and its structural analogs by a newly isolated Agrobacterium sp. PH-08. To assess the biodegradation potential of newly isolated Agrobacterium sp. PH-08, various substrates were evaluated as sole carbon sources in growth and
Markus Hauck et al.
Annals of botany, 103(1), 13-22 (2008-11-04)
Many species of lichen-forming fungi contain yellow or orange extracellular pigments belonging to the dibenzofurans (usnic acid), anthraquinones (e.g. parietin) or pulvinic acid group. These pigments are all equally efficient light screens, leading us to question the potential ecological and
Jing Pan et al.
Bulletin of environmental contamination and toxicology, 89(6), 1240-1246 (2012-09-26)
The concentrations of polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/Fs), dioxin-like polychlorinated biphenyls (PCBs), and polychlorinated naphthalenes (PCNs) were measured in two sediment cores collected from Jiaozhou Bay. The concentrations of PCDD/Fs, dioxin-like PCBs, and PCNs in the cores were in the range of
Qingyao Shou et al.
Journal of natural products, 75(9), 1612-1617 (2012-09-01)
In an effort to identify new anti-inflammatory and antibacterial agents with potential application in wound healing, five new dibenzofurans, 1,3,7,9-tetrahydroxy-2,8-dimethyl-4,6-di(2-methylbutanoyl)dibenzofuran (1), 1,3,7,9-tetrahydroxy-2,8-dimethyl-4-(2-methylbutanoyl)-6-(2-methylpropionyl)dibenzofuran (2), 1,3,7,9-tetrahydroxy-2,8-dimethyl-4,6-di(2-methylpropionyl)dibenzofuran (3), 1,3,7,9-tetrahydroxy-4,6-dimethyl-2-(2-methylbutanoyl)-8-(2-methylpropionyl)dibenzofuran (4), and 1,3,7,9-tetrahydroxy-4,6-dimethyl-2,8-di(2-methylpropionyl)dibenzofuran (5), were isolated from the leaves of Pilidiostigma glabrum together with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![Benzo[b]fluoranthene 98%](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)


